Yonsei Med J.
2020 Mar;61(3):243-250. 10.3349/ymj.2020.61.3.243.
Signal Detection of Adverse Events Following Pneumococcal Vaccines from the Korea Adverse Event Reporting System Database, 2005–2016
- Affiliations
-
- 1Department of Pharmaceutical Medicine and Regulatory Sciences, Yonsei University Graduate School, Seoul, Korea. minspark@yuhs.ac
- 2School of Pharmacy, Sungkyunkwan University, Suwon, Korea. shin.jy@skku.edu
- 3College of Nursing and Health, Kongju National University, Gongju, Korea.
- 4Department of Pediatrics, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2470921
- DOI: http://doi.org/10.3349/ymj.2020.61.3.243
Abstract
- PURPOSE
We aimed to analyze the surveillance reports of adverse events (AEs) due to different types of pneumococcal vaccines, in addition to detecting and validating signals of pneumococcal vaccines by comparing AEs with labels.
MATERIALS AND METHODS
We analyzed the percentages of AEs according to vaccine type [pneumococcal polysaccharide vaccines (PPSVs) and pneumococcal conjugate vaccines (PCVs)] in children and adults using data from the Korea Adverse Event Reporting System (KAERS) database from 2005 to 2016. A signal was defined as an AE that met all three indices of data mining: proportional reporting ratio (PRR), reporting odds ratio (ROR), and information component (IC). We validated the detected signals by calculating sensitivity, specificity, as well as positive and negative predictive values of the signals against label information.
RESULTS
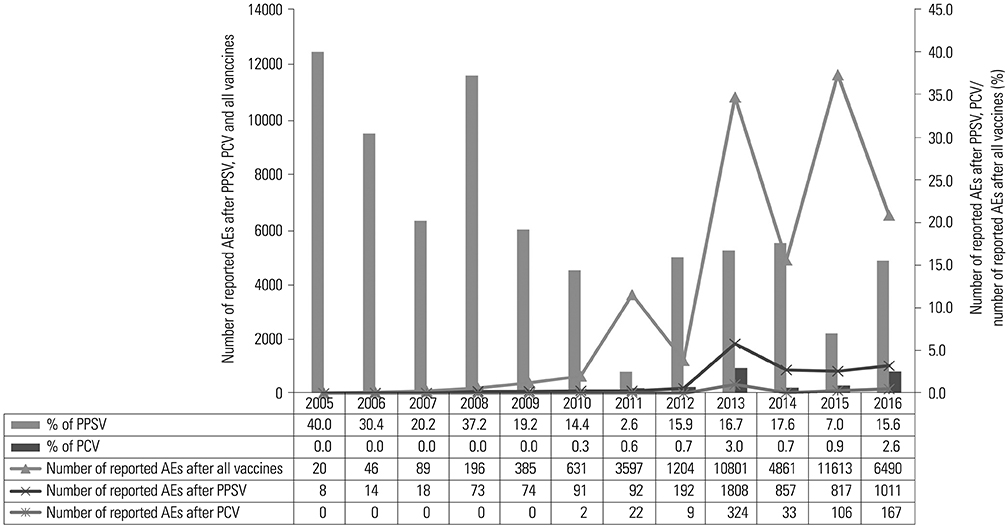
Of the 39933 AE reports on vaccination, 5718 (7.0%) were related to pneumococcal vaccine. The most frequent AE after vaccination with PPSV was fever (23.9%) in children and injection-site reaction in adults. The most frequent AE after vaccination with PCV in children was pharyngitis (26.2%). In total, 13 AEs met all three indices for signal detection. Among these, hypotension, apathy, sepsis, and increased serum glutamic oxaloacetic transaminase level were not listed on vaccine labels. In validation analysis, PRR and ROR performed slightly better than IC for adults who were vaccinated with PPSVs.
CONCLUSION
Overall, 13 new signals of PPSVs, including four signals not listed on the labels, were detected. Further research based on additional AE reports is required to confirm the validity of these signals for children.
MeSH Terms
Figure
Reference
-
1. Statistic Korea. Causes of death statistics in 2017. accessed on 2019 September 19. Available at: http://kostat.go.kr/portal/eng/pressReleases/8/10/index.board?bmode=read&bSeq=&aSeq=371140&pageNo=1&rowNum=10&navCount=10&currPg=&searchInfo=&sTarget=title&sTxt=.2. File TM Jr. Streptococcus pneumoniae and community-acquired pneumonia: a cause for concern. Am J Med. 2004; 117 Suppl 3A:39S–50S.
Article3. Schrag SJ, Beall B, Dowell SF. Limiting the spread of resistant pneumococci: biological and epidemiologic evidence for the effectiveness of alternative interventions. Clin Microbiol Rev. 2000; 13:588–601.
Article4. Whitney CG, Farley MM, Hadler J, Harrison LH, Lexau C, Reingold A, et al. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. N Engl J Med. 2000; 343:1917–1924.
Article5. McCormick AW, Whitney CG, Farley MM, Lynfield R, Harrison LH, Bennett NM, et al. Geographic diversity and temporal trends of antimicrobial resistance in Streptococcus pneumoniae in the United States. Nat Med. 2003; 9:424–430.
Article6. Kong Y, Zhang W, Jiang Z, Wang L, Li C, Li Y, et al. Immunogenicity and safety of a 23-valent pneumococcal polysaccharide vaccine in Chinese healthy population aged >2 years: a randomized, double-blinded, active control, phase III trial. Hum Vaccin Immunother. 2015; 11:2425–2433.
Article7. Marra F, Vadlamudi NK. Efficacy and safety of the pneumococcal conjugate-13 valent vaccine in adults. Aging Dis. 2019; 10:404–418.
Article8. Kim ES, Shin JK, Oh HK. Elderly immunization program against invasive pneumococcal disease in Korea, 2013. Public Health Wkly Rep. 2014; 7:182–186.9. Korea Centers for Disease Control and Previention. Korea National Immunization Program. 2014. accessed on 2019 September 19. Available at: https://nip.cdc.go.kr/irgd/support.do?service=getNewsView&strNum=2&PROSEQNUM=305.10. National Institute of Food and Drug Safety Evaluation. 2017 annual report of national lot release. accessed on 2019 September 19. Available at: https://www.mfds.go.kr/brd/m_231/view.do?seq=32914.11. Centers for Disease Control and Prevention (CDC).Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012; 61:816–819.12. World Health Organization. Information sheet observed rate of vaccine reactions pneumococcal vaccine. accessed on 2019 September 19. Available at: https://www.who.int/vaccine_safety/initiative/tools/vaccinfosheets/en/.13. Jackson LA, Gurtman A, van Cleeff M, Jansen KU, Jayawardene D, Devlin C, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine compared to a 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naive adults. Vaccine. 2013; 31:3577–3584.
Article14. Jackson LA, Gurtman A, Rice K, Pauksens K, Greenberg RN, Jones TR, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults 70 years of age and older previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine. 2013; 31:3585–3593.
Article15. Food, Medicine and Healthcare Administration and Control Authority of Ethiopia. Guideline for adverse drug events monitoring (pharmacovigilance). 3rd ed. Addis Ababa: Food, Medicine and Healthcare Administration and Control Authority of Ethiopia;2014.16. Bate A, Lindquist M, Edwards IR, Olsson S, Orre R, Lansner A, et al. A Bayesian neural network method for adverse drug reaction signal generation. Eur J Clin Pharmacol. 1998; 54:315–321.
Article17. Chan IS, Zhang Z. Test-based exact confidence intervals for the difference of two binomial proportions. Biometrics. 1999; 55:1202–1209.
Article18. Merck Sharp and Dohme Corporation. PNEUMOVAX®23 (pneumococcal vaccine polyvalent) package insert. accessed on 2016 October 2. Available at: http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM257088.pdf.19. Merck Sharp and Dohme Corporation. PRODIAX®23 (pneumococcal vaccine polyvalent) package insert. accessed on 2019 September 19. Available at: https://www.msd-korea.com/assets/pdf/products/PRODIAX23.pdf.20. Pfizer. Prevenar®13 (pneumococcal conjugate vaccine) package insert. accessed on 2019 September 19. Available at: https://www.pfizer.co.kr/products/prevenar%C2%AE13-%ED%94%84%EB%A6%AC%EB%B2%A0%EB%82%98%C2%AE13%EC%A3%BC.21. GlaxoSmithKline. Synflorix® (pneumococcal conjugate vaccine) package insert. accessed on 2019 September 19. Available at: https://nedrug.mfds.go.kr/pbp/CCBBB01/getItemDetail?itemSeq=201002299.22. Miller ER, Moro PL, Cano M, Lewis P, Bryant-Genevier M, Shimabukuro TT. Post-licensure safety surveillance of 23-valent pneumococcal polysaccharide vaccine in the Vaccine Adverse Event Reporting System (VAERS), 1990-2013. Vaccine. 2016; 34:2841–2846.
Article23. Törling J, Hedlund J, Konradsen HB, Ortqvist A. Revaccination with the 23-valent pneumococcal polysaccharide vaccine in middle-aged and elderly persons previously treated for pneumonia. Vaccine. 2003; 22:96–103.
Article24. Burwen DR, La Voie L, Braun MM, Houck P, Ball R. Evaluating adverse events after vaccination in the Medicare population. Pharmacoepidemiol Drug Saf. 2007; 16:753–761.
Article25. Brivet F, Herer B, Fremaux A, Dormont J, Tchernia G. Fatal post-splenectomy pneumococcal sepsis despite pneumococcal vac-cine and penicillin prophylaxis. Lancet. 1984; 2:356–357.
Article26. Kerner A, Avizohar O, Sella R, Bartha P, Zinder O, Markiewicz W, et al. Association between elevated liver enzymes and C-reactive protein: possible hepatic contribution to systemic inflammation in the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2005; 25:193–197.27. Korea Centers for Disease Control and Previention. Newsletter of National Immunization Program 2016. accessed on 2019 September 19. Available at: https://nip.cdc.go.kr/irgd/support.do?MnLv1=1.28. Shimabukuro TT, Nguyen M, Martin D, DeStefano F. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2015; 33:4398–4405.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Class-Effect Study of Vaccine Signal Detection Using Korea Adverse Event Reporting System Database
- Updates on Vaccine Safety and Post-Licensure Surveillance for Adverse Events Following Immunization in South Korea, 2005–2017
- Signal Detection for Adverse Events of Finasteride Using Korea Adverse Event Reporting System (KAERS) Database
- Signal Detection and Safety Information Generation of Aripiprazole in Spontaneous Adverse Event Reports Database
- Analysis of Important Medical Adverse Events and Signals Related with Cyclosporine and Tacrolimus Using the FDA Adverse Event Reporting System (FAERS) Database