J Breast Cancer.
2019 Dec;22(4):647-652. 10.4048/jbc.2019.22.e50.
Clinical Effects of Hypomethylating Agents in Patients with Newly Diagnosed Myelodysplastic Syndrome Who Received DNA-Damaging Chemotherapy for Metastatic Breast Cancer
- Affiliations
-
- 1Department of Oncology/Hematology, Kyungpook National University Chilgok Hospital, Kyungpook National University Cancer Research Institute, Kyungpook National University School of Medicine, Daegu, Korea. yschae@knu.ac.kr
- KMID: 2470901
- DOI: http://doi.org/10.4048/jbc.2019.22.e50
Abstract
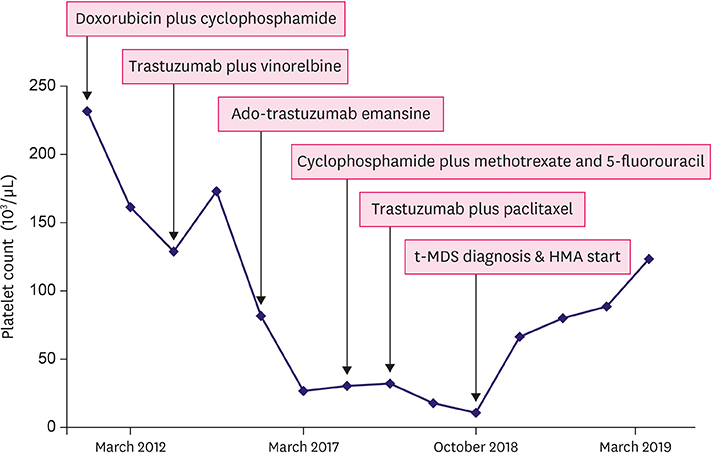
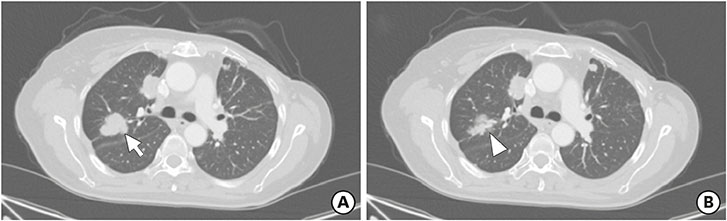
- The cumulative risk of therapy-related myelodysplastic syndrome (t-MDS) in breast cancer patients exposed to chemotherapy and/or radiotherapy is significantly high compared to that in other cancer patients. This report reviews the use of hypomethylating agents (HMAs) to treat a 57-year-old woman newly diagnosed with MDS during palliative chemotherapy for metastatic breast cancer. Over a period of 6 years, the patient received several DNA-damaging chemotherapeutics including doxorubicin, cyclophosphamide, and paclitaxel. Repeated thrombocytopenia was the main reason for suspecting secondary hematologic malignancy. She was diagnosed with t-MDS based on bone marrow examination and her treatment history for breast cancer. While azacitidine was originally administered to stabilize MDS, it also stabilized the patient's lung and lymph node metastases without any major toxicity. Therefore, the current case highlights the promising effects of HMAs for treating t-MDS following heavily pretreated breast cancer.
MeSH Terms
-
Azacitidine
Bone Marrow Examination
Breast Neoplasms*
Breast*
Cyclophosphamide
DNA Methylation
Doxorubicin
Drug Therapy*
Female
Hematologic Neoplasms
Humans
Lung
Lymph Nodes
Middle Aged
Myelodysplastic Syndromes*
Neoplasm Metastasis
Paclitaxel
Radiotherapy
Thrombocytopenia
Azacitidine
Cyclophosphamide
Doxorubicin
Paclitaxel
Figure
Reference
-
1. Shenolikar R, Durden E, Meyer N, Lenhart G, Moore K. Incidence of secondary myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) in patients with ovarian or breast cancer in a real-world setting in the United States. Gynecol Oncol. 2018; 151:190–195.
Article2. Kaplan HG, Malmgren JA, Atwood MK. Increased incidence of myelodysplastic syndrome and acute myeloid leukemia following breast cancer treatment with radiation alone or combined with chemotherapy: a registry cohort analysis 1990–2005. BMC Cancer. 2011; 11:260.
Article3. El-Fattah MA. Clinical features and outcomes of 666 cases with therapy-related myelodysplastic syndrome (t-MDS). Indian J Hematol Blood Transfus. 2018; 34:83–90.
Article4. Merlo A, Herman JG, Mao L, Lee DJ, Gabrielson E, Burger PC, et al. 5′ CpG island methylation is associated with transcriptional silencing of the tumour suppressor p16/CDKN2/MTS1 in human cancers. Nat Med. 1995; 1:686–692.
Article5. Glasspool RM, Teodoridis JM, Brown R. Epigenetics as a mechanism driving polygenic clinical drug resistance. Br J Cancer. 2006; 94:1087–1092.
Article6. de Sousa E Melo F, Colak S, Buikhuisen J, Koster J, Cameron K, de Jong JH, et al. Methylation of cancer-stem-cell-associated Wnt target genes predicts poor prognosis in colorectal cancer patients. Cell Stem Cell. 2011; 9:476–485.
Article7. Linnekamp JF, Butter R, Spijker R, Medema JP, van Laarhoven HW. Clinical and biological effects of demethylating agents on solid tumours - a systematic review. Cancer Treat Rev. 2017; 54:10–23.
Article8. Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Solé F, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012; 120:2454–2465.
Article9. Malmgren JA, Calip GS, Pyott SM, Atwood MK, Kaplan HG. Therapy-related myelodysplastic syndrome following primary breast cancer. Leuk Res. 2016; 47:178–184.
Article10. Larson RA. Therapy-related myeloid neoplasms. Haematologica. 2009; 94:454–459.
Article11. Maung SW, Burke C, Hayde J, Walshe J, McDermott R, Desmond R, et al. A review of therapy-related myelodysplastic syndromes and acute myeloid leukaemia (t-MDS/AML) in Irish patients: a single centre experience. Hematology. 2017; 22:341–346.
Article12. Ando M, Narabayashi M, Watanabe T, Kamiya Y, Togitani K, Tanosaki R, et al. Therapy-related leukemia and myelodysplastic syndrome in breast cancer patients treated with cyclophosphamide or anthracyclines. Jpn J Clin Oncol. 1999; 29:28–32.
Article13. Cruijsen M, Lübbert M, Wijermans P, Huls G. Clinical results of hypomethylating agents in AML treatment. J Clin Med. 2014; 4:1–17.
Article14. Izbicka E, Davidson KK, Lawrence RA, MacDonald JR, Von Hoff DD. 5,6-Dihydro-5′-azacytidine (DHAC) affects estrogen sensitivity in estrogen-refractory human breast carcinoma cell lines. Anticancer Res. 1999; 19:1293–1298.15. Stone A, Valdés-Mora F, Gee JM, Farrow L, McClelland RA, Fiegl H, et al. Tamoxifen-induced epigenetic silencing of oestrogen-regulated genes in anti-hormone resistant breast cancer. PLoS One. 2012; 7:e40466.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Enhancement of Radiosensitivity by DNA Hypomethylating Drugs through Apoptosis and Autophagy in Human Sarcoma Cells
- A Study Tumoric Topoisomerase II alpha enzyme, c-erb B-2oncoprotein, and P-glycoprotein Expression as an Indicator of Therapeutic Failure in Breast Cancer Patients Received Chemotherapy
- Diagnosis and Treatment of HER2-Positive Breast Cancer
- New agents for the treatment of myelodysplastic syndromes
- Mutations in genes affecting DNA methylation enhances responses to decitabine in patients with myelodysplastic syndrome