J Breast Cancer.
2020 Feb;23(1):100-106. 10.4048/jbc.2020.23.e1.
Acute Lymphoblastic Leukemia in a Patient Treated with Letrozole and Palbociclib
- Affiliations
-
- 1Division of Hematology-Medical Oncology, Department of Internal Medicine, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul National University College of Medicine, Seoul, Korea. jinhyunpak@gmail.com
- 2Department of Laboratory Medicine, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Pathology, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2470886
- DOI: http://doi.org/10.4048/jbc.2020.23.e1
Abstract
- Palbociclib, in conjunction with endocrine therapy, has been approved for the treatment of patients with advanced breast cancer. The common hematological toxicities associated with palbociclib are leukopenia and neutropenia. However, hematological malignancies have not been reported for palbociclib treatment. Here, for the first time, we present a case of acute lymphoblastic leukemia that was diagnosed in a patient undergoing treatment with letrozole and palbociclib for metastatic breast cancer. This case emphasizes the need for long term follow up of patients treated with palbociclib.
Keyword
MeSH Terms
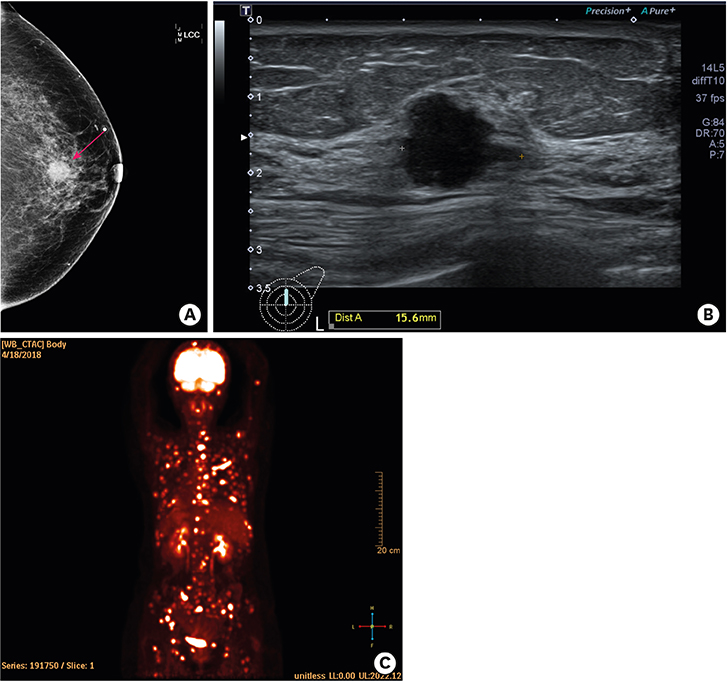
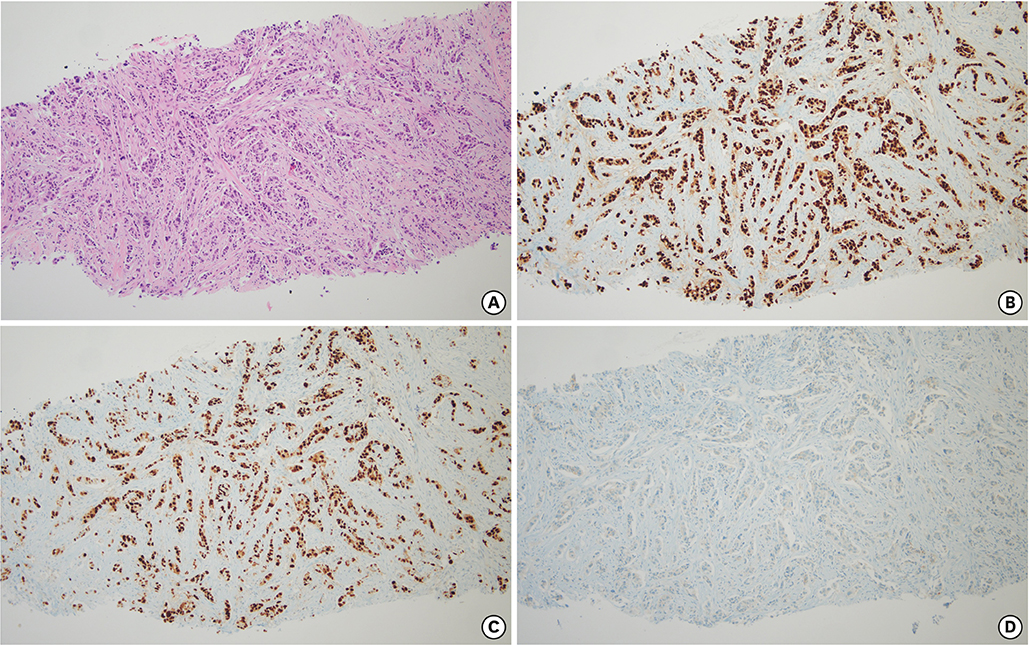
Figure
Reference
-
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108.
Article2. Nadji M, Gomez-Fernandez C, Ganjei-Azar P, Morales AR. Immunohistochemistry of estrogen and progesterone receptors reconsidered: experience with 5,993 breast cancers. Am J Clin Pathol. 2005; 123:21–27.
Article3. Rugo HS, Rumble RB, Macrae E, Barton DL, Connolly HK, Dickler MN, et al. Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Society of Clinical Oncology guideline. J Clin Oncol. 2016; 34:3069–3103.
Article4. Chlebowski RT. Changing concepts of hormone receptor-positive advanced breast cancer therapy. Clin Breast Cancer. 2013; 13:159–166.
Article5. Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016; 375:1925–1936.
Article6. Hamilton E, Infante JR. Targeting CDK4/6 in patients with cancer. Cancer Treat Rev. 2016; 45:129–138.
Article7. Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro . Breast Cancer Res. 2009; 11:R77.
Article8. Shapiro GI. Cyclin-dependent kinase pathways as targets for cancer treatment. J Clin Oncol. 2006; 24:1770–1783.
Article9. Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016; 17:425–439.
Article10. Diéras V, Rugo HS, Schnell P, Gelmon K, Cristofanilli M, Loi S, et al. Long-term pooled safety analysis of palbociclib in combination with endocrine therapy for HR+/HER2- advanced breast cancer. J Natl Cancer Inst. 2019; 111:419–430.
Article11. Matushansky I, Radparvar F, Skoultchi AI. Reprogramming leukemic cells to terminal differentiation by inhibiting specific cyclin-dependent kinases in G1 . Proc Natl Acad Sci U S A. 2000; 97:14317–14322.
Article12. Müller PR, Meier R, Hirt A, Bodmer JJ, Janic D, Leibundgut K, et al. Nuclear lamin expression reveals a surprisingly high growth fraction in childhood acute lymphoblastic leukemia cells. Leukemia. 1994; 8:940–945.13. Sherr CJ, Beach D, Shapiro GI. Targeting CDK4 and CDK6: from discovery to therapy. Cancer Discov. 2016; 6:353–367.
Article14. Ribera JM. Therapy-related acute lymphoblastic leukemia. Haematologica. 2018; 103:1581–1583.
Article15. Aldoss I, Stiller T, Tsai NC, Song JY, Cao T, Bandara NA, et al. Therapy-related acute lymphoblastic leukemia has distinct clinical and cytogenetic features compared to de novo acute lymphoblastic leukemia, but outcomes are comparable in transplanted patients. Haematologica. 2018; 103:1662–1668.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Precursor B-Cell Acute Lymphoblastic Leukemia in Two Patients with a History of Cytotoxic Therapy
- A case of acute lymphoblastic leukemia complicating neuroblastoma in remission
- A case of bone marrow necrosis in acute lymphoblastic leukemia
- Meningeal Chloromain in Acute Lymphoblastic Leukemia
- Bilateral Nephromegaly as a Presenting Symptom of Acute Lymphoblastic Leukemia