Clin Exp Vaccine Res.
2020 Jan;9(1):8-14. 10.7774/cevr.2020.9.1.8.
Cold chain time- and temperature-controlled transport of vaccines: a simulated experimental study
- Affiliations
-
- 1Faculty of Pharmacy, Quest International University Perak, Ipoh, Malaysia. yenloong.lean@qiup.edu.my
- 2Department of Pharmacy, National University Health System, Singapore, Singapore.
- 3Faculty of Pharmacy, Universiti Teknologi MARA, Kepala Batas, Malaysia.
- 4Faculty of Pharmacy, University of Cyberjaya, Cyberjaya, Malaysia.
- 5College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al-Kharj, Saudi Arabia.
- 6College of Pharmacy, University of Science and Technology of Fujairah, Fujairah, United Arab Emirates.
- 7PAPRSB Institute of Health Sciences, Universiti Brunei Darussalam, Gadong, Brunei Darussalam. ming.long@bath.edu
- KMID: 2470833
- DOI: http://doi.org/10.7774/cevr.2020.9.1.8
Abstract
- PURPOSE
The objective of this research was to examine the cold chain temperature maintenance for the supply of vaccines and other biological products by pharmaceutical wholesaler.
MATERIALS AND METHODS
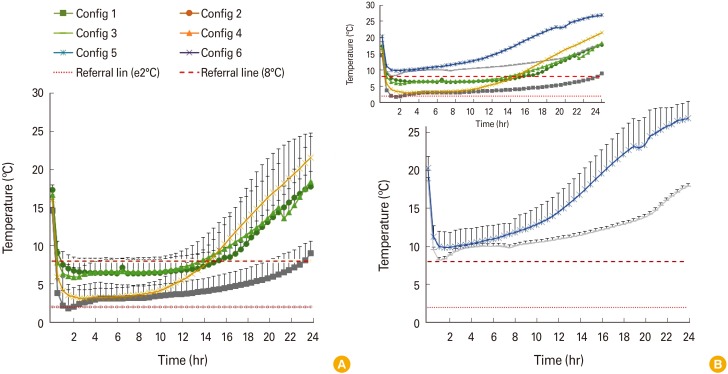
In this study, six configurations using cold vaccine boxes or bags made with different materials, with and without insulation, of different sizes, and number of coolant-packs were used to simulate the configuration used by the pharmaceutical wholesalers for transportation of vaccine. Model vaccines (vial, n=10) were packed using these six configurations which then stored in an incubator at 38℃ and monitored for 24 hours. Each configuration was tested repeatedly for 5 times.
RESULTS
In term of compliance to 2℃-8℃, four out of six tested configurations are effective in cold chain transportation. The effectiveness is highly dependent on the type of passive containers used, size of cold boxes, insulation, and number of coolant-packs. The configuration with a larger polystyrene foam box with five coolant-packs maintained the required temperature up to 23 hours. In contrast, configurations using a polystyrene foam box with four coolant-packs and a large vaccine cold box with two coolant-packs failed to reach below 8℃ throughout the 24 hours.
CONCLUSION
Packaging method, the material and size of the container could have a direct impact on the effectiveness of cold chain temperature maintenance. Polystyrene foam box, cold box with polyethylene interior lining and polypropylene insulation, a cooler bag with proper number of ice packs could be effectively used for transportation of vaccines within their respective transportation duration allowance.
MeSH Terms
Figure
Reference
-
1. Department of Immunization, Vaccines and Biologicals, World Health Organization. Study protocol for temperature monitoring in the vaccine cold chain [Internet]. Geneva: World Health Organization;2011. cited 2019 Jun 5. Available from: https://apps.who.int/iris/bitstream/handle/10665/70752/WHO_IVB_05.01_REV.1_eng.pdf.2. World Health Organization. How to calculate vaccine volumes and cold chain capacity requirements (WHO/IVB/ 17.06) [Internet]. Geneva: World Health Organization;2017. cited 2019 Jun 6. Available from: https://www.who.int/immunization/documents/control/who_ivb_17.06/en/.3. Lloyd J, Cheyne J. The origins of the vaccine cold chain and a glimpse of the future. Vaccine. 2017; 35:2115–2120. PMID: 28364918.
Article4. Sykes C. Time- and temperature-controlled transport: supply chain challenges and solutions. P T. 2018; 43:154–170. PMID: 29491697.5. Hibbs BF, Miller E, Shi J, Smith K, Lewis P, Shimabukuro TT. Safety of vaccines that have been kept outside of recommended temperatures: reports to the Vaccine Adverse Event Reporting System (VAERS), 2008-2012. Vaccine. 2018; 36:553–558. PMID: 29248264.
Article6. Hanson CM, George AM, Sawadogo A, Schreiber B. Is freezing in the vaccine cold chain an ongoing issue?: a literature review. Vaccine. 2017; 35:2127–2133. PMID: 28364920.
Article7. Program for Appropriate Technology in Health; World Health Organization; Health Systems Research Institute; Mahidol University. An assessment of vaccine supply chain and logistics systems in Thailand. Seattle, WA: Program for Appropriate Technology in Health;2011. cited 2019 Jun 6. Available from: http://path.azureedge.net/media/documents/TS_opt_vac_sup_thai.pdf.8. Kartoglu U, Nelaj E, Maire D. Improving temperature monitoring in the vaccine cold chain at the periphery: an intervention study using a 30-day electronic refrigerator temperature logger (Fridge-tag). Vaccine. 2010; 28:4065–4072. PMID: 20398615.
Article9. Nelson C, Froes P, Dyck AM, et al. Monitoring temperatures in the vaccine cold chain in Bolivia. Vaccine. 2007; 25:433–437. PMID: 17000036.
Article10. Bekcic S, Kelecevic N, Marinkovic V, Tasic L, Krajnovic D. Developing a quality management tool for preparing Good Distribution Practice audit of pharmaceutical contract vaccine distributor. Indian J Pharm Educ Res. 2015; 49:174–182.11. Kartoglu U, Vesper J, Teras H, Reeves T. Experiential and authentic learning approaches in vaccine management. Vaccine. 2017; 35:2243–2251. PMID: 28364938.
Article12. World Health Organization. Vaccine carrier: performance, quality and safety performance specification (WHO/PQS/E004/VC01.2) [Internet]. Geneva: World Health Organization;2010. cited 2019 Jun 5. Available from: http://apps.who.int/immunization_standards/vaccine_quality/pqs_catalogue/catdocumentation.aspx?id_cat=18.13. World Health Organization. Vaccine cold box: performance, quality and safety performance specification (WHO/PQS/E004/CB01.3) [Internet]. Geneva: World Health Organization;2012. cited 2019 Jun 5. Available from: http://apps.who.int/immunization_standards/vaccine_quality/pqs_catalogue/catdocumentation.aspx?id_cat=18.14. World Health Organization. Large capacity vaccine cold box: performance, quality and safety performance specification (WHO/PQS/E004/CB02.1) [Internet]. Geneva: World Health Organization;2012. cited 2019 Jun 5. Available from: http://apps.who.int/immunization_standards/vaccine_quality/pqs_catalogue/catdocumentation.aspx?id_cat=18.15. Center for Drug Evaluation and Research, Food and Drug Administration. Guidance for industry: waiver of in vivo bioavailability and bioequivalence studies for immediate-release solid oral dosage forms based on a Biopharmaceutics Classification System [Internet]. Silver Spring, MD: Food and Drug Administration;2000. cited 2010 Sep 11. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070246.pdf.16. Vertzoni M, Symillides M, Iliadis A, Nicolaides E, Reppas C. Comparison of simulated cumulative drug versus time data sets with indices. Eur J Pharm Biopharm. 2003; 56:421–428. PMID: 14602186.
Article17. Murhekar MV, Dutta S, Kapoor AN, et al. Frequent exposure to suboptimal temperatures in vaccine cold-chain system in India: results of temperature monitoring in 10 states. Bull World Health Organ. 2013; 91:906–913. PMID: 24347729.
Article18. Ahmed A, Lee KS, Bukhsh A, et al. Outbreak of vaccine-preventable diseases in Muslim majority countries. J Infect Public Health. 2018; 11:153–155. PMID: 28988775.
Article19. Desai SN, Kamat D. Closing the global immunization gap: delivery of lifesaving vaccines through innovation and technology. Pediatr Rev. 2014; 35:e32–e40. PMID: 24986933.
Article20. Shimabukuro TT, Nguyen M, Martin D, DeStefano F. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2015; 33:4398–4405. PMID: 26209838.
Article21. Matthias DM, Robertson J, Garrison MM, Newland S, Nelson C. Freezing temperatures in the vaccine cold chain: a systematic literature review. Vaccine. 2007; 25:3980–3986. PMID: 17382434.
Article22. Yauba S, Joelle S, Jude N, Tracy BO, Marie K. Temperature monitoring in the vaccine cold chain in Cameroon. J Vaccines Vaccin. 2018; 9:2.
Article23. Njuguna MW, Mairura CJ, Ombui K. Influence of cold chain supply logistics on the safety of vaccines: a case of pharmaceutical distributors in Nairobi County. Int J Sci Res Publ. 2015; 5:178–196.24. Kitamura T, Bouakhasith V, Phounphenghack K, et al. Assessment of temperatures in the vaccine cold chain in two provinces in Lao People's Democratic Republic: a cross-sectional pilot study. BMC Res Notes. 2018; 11:261. PMID: 29703228.
Article25. Breakwell L, Anga J, Dadari I, Sadr-Azodi N, Ogaoga D, Patel M. Evaluation of storing hepatitis B vaccine outside the cold chain in the Solomon Islands: identifying opportunities and barriers to implementation. Vaccine. 2017; 35:2770–2774. PMID: 28431814.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inspection and Evaluation of Blood Cold Chain
- The Vaccine Cold Chain in North Korea: Assessing the Capacity to Store Routine Vaccines and Potential to Support Pandemic Vaccination Activities
- Evaluation of precipitation time of the aluminum salts adsorbed potentially frozen vaccines used in the Polish National Immunization Schedule for their pre-qualification before the administration
- Cold-associated skin disorders
- Cold-stress Test Involving Finger Skin Temperature Measurement for Evaluation of Raynaud's Disease and Nonspecific Cold Sensitive Patients