Transl Clin Pharmacol.
2019 Dec;27(4):134-140. 10.12793/tcp.2019.27.4.134.
Pharmacokinetic comparison between fixed-dose combination of fimasartan/amlodipine 60/10 mg and the corresponding loose combination through partial replicated crossover study in healthy subjects
- Affiliations
-
- 1Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine and Hospital, Seoul 03080, Republic of Korea. leejh413@snu.ac.kr
- KMID: 2467967
- DOI: http://doi.org/10.12793/tcp.2019.27.4.134
Abstract
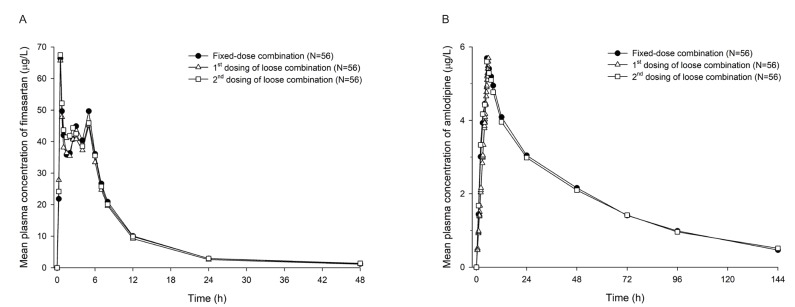
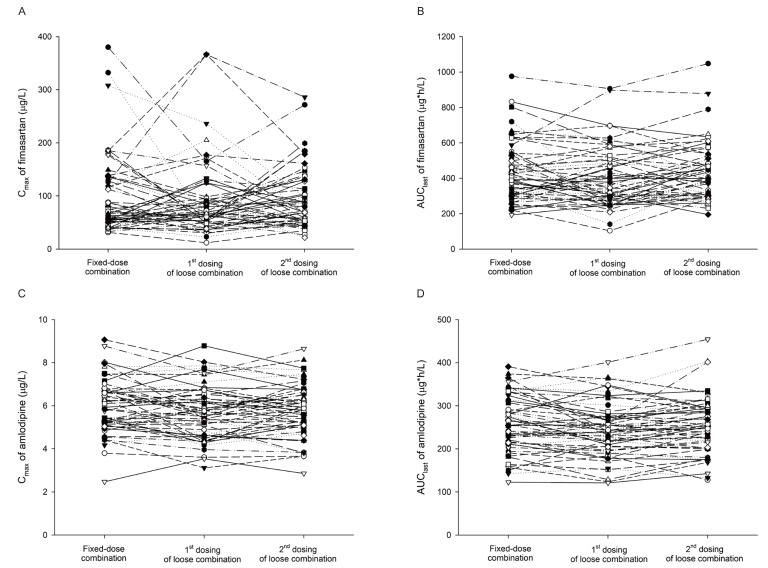
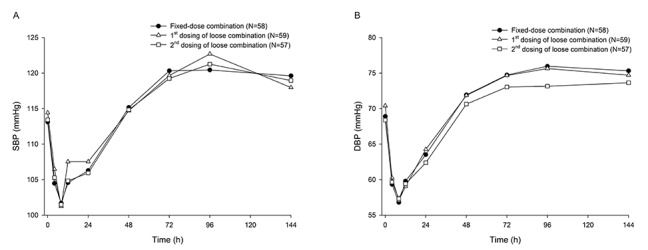
- Combination therapies of antihypertensive drugs are recommended in cases where hypertension is not controlled by monotherapy. This study aimed to compare the pharmacokinetics (PKs) between fixed-dose combination (FDC) of fimasartan/amlodipine 60/10 mg and the corresponding loose combination. Because of the high intra-subject variability for maximum plasma concentration (C(max)) of fimasartan, a randomized, open-label, 3×3 partial replicated crossover design was adopted. Subjects received a single dose of FDC of fimasartan/amlodipine 60/10 mg or the corresponding loose combination in each period. Blood samples for PK analysis were collected up to 48 hours for fimasartan and 144 hours for amlodipine, respectively. Geometric mean ratios (GMRs) and its 90% confidence intervals (CIs) of the FDC to the loose combination for C(max) and area under the concentration-time curve from time 0 to the last quantifiable time point (AUC(last)) were calculated. Sixty healthy subjects were randomized, and 57 subjects completed the study. The concentration-time profiles of fimasartan and amlodipine were similar between the FDC and loose combination. The GMRs (90% CIs) of the FDC to the loose combination for C(max) and AUC(last) were 1.0440 (0.9202-1.1844) and 1.0412 (0.9775-1.1090) for fimasartan, and 1.0430 (1.0156-1.0711) and 1.0339 (1.0055-1.0631) for amlodipine, respectively. The GMRs and its 90% CIs for C(max) and AUC(last) of fimasartan and amlodipine were included not only in the scaled bioequivalence criteria but also in the conventional bioequivalence criteria. In conclusion, FDC of fimasartan/amlodipine 60/10 mg showed comparable PK profiles with the corresponding loose combination, which suggests their bioequivalence.
Keyword
MeSH Terms
Figure
Reference
-
1. Kannel WB. Blood pressure as a cardiovascular risk factor: prevention and treatment. JAMA. 1996; 275:1571–1576. PMID: 8622248.
Article2. Lee HY, Shin J, Kim GH, Park S, Ihm SH, Kim HC, et al. 2018 Korean Society of Hypertension Guidelines for the management of hypertension: part II-diagnosis and treatment of hypertension. Clin Hypertens. 2019; 25:20. DOI: 10.1186/s40885-019-0124-x. PMID: 31388453.
Article3. Wald DS, Law M, Morris JK, Bestwick JP, Wald NJ. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med. 2009; 122:290–300. DOI: 10.1016/j.amjmed.2008.09.038. PMID: 19272490.
Article4. Chrysant SG. The role of Angiotensin receptor blocker and calcium channel blocker combination therapy in treating hypertension: focus on recent studies. Am J Cardiovasc Drugs. 2010; 10:315–320. DOI: 10.2165/11538850-000000000-00000. PMID: 20860414.5. da Silva PM. Efficacy of fixed-dose combination therapy in the treatment of patients with hypertension: focus on amlodipine/valsartan. Clin Drug Investig. 2010; 30:625–641. DOI: 10.2165/11538440-000000000-00000.6. Ishimitsu T, Ohno E, Nakano N, Furukata S, Akashiba A, Minami J, et al. Combination of angiotensin II receptor antagonist with calcium channel blocker or diuretic as antihypertensive therapy for patients with chronic kidney disease. Clin Exp Hypertens. 2011; 33:366–372. DOI: 10.3109/10641963.2010.503299. PMID: 21797795.
Article7. Yi S, Kim TE, Yoon SH, Cho JY, Shin SG, Jang IJ, et al. Pharmacokinetic interaction of fimasartan, a new angiotensin II receptor antagonist, with amlodipine in healthy volunteers. J Cardiovasc Pharmacol. 2011; 57:682–689. DOI: 10.1097/FJC.0b013e31821795d0. PMID: 21394036.
Article8. Kang WY, Seong SJ, Ohk B, Gwon MR, Kim BK, Cho S, et al. Pharmacokinetic and bioequivalence study comparing a fimasartan/rosuvastatin fixed-dose combination with the concomitant administration of fimasartan and rosuvastatin in healthy subjects. Drug Des Devel Ther. 2018; 12:3607–3615. DOI: 10.2147/dddt.S161917.
Article9. Ghim JL, Paik SH, Hasanuzzaman M, Chi YH, Choi HK, Kim DH, et al. Absolute bioavailability and pharmacokinetics of the angiotensin II receptor antagonist fimasartan in healthy subjects. J Clin Pharmacol. 2016; 56:576–580. DOI: 10.1002/jcph.618. PMID: 26272450.
Article10. Chi YH, Lee H, Paik SH, Lee JH, Yoo BW, Kim JH, et al. Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Fimasartan Following Single and Repeated Oral Administration in the Fasted and Fed States in Healthy Subjects. Am J Cardiovasc Drugs. 2011; 11:335–346. DOI: 10.2165/11593840-000000000-00000. PMID: 21910510.
Article11. Abernethy DR. Pharmacokinetics and pharmacodynamics of amlodipine. Cardiology. 1992; 80 Suppl 1:31–36. DOI: 10.1159/000175050. PMID: 1534713.
Article12. Son M, Guk J, Kim Y, Woo Chae D, Heo YA, Soh D, et al. Pharmacokinetic Interaction Between Rosuvastatin, Telmisartan, and Amlodipine in Healthy Male Korean Subjects: A Randomized, Open-label, Multipledose, 2-period Crossover Study. Clin Ther. 2016; 38:1845–1857. DOI: 10.1016/j.clinthera.2016.06.011. PMID: 27422590.
Article13. Bangalore S, Kamalakkannan G, Parkar S, Messerli FH. Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med. 2007; 120:713–719. DOI: 10.1016/j.amjmed.2006.08.033. PMID: 17679131.
Article14. Stankus V, Hemmelgarn B, Campbell NR, Chen G, McAlister FA, Tsuyuki RT. Reducing costs and improving hypertension management. Can J Clin Pharmacol. 2009; 16:e151–e155. PMID: 19193969.15. Sherrill B, Halpern M, Khan S, Zhang J, Panjabi S. Single-pill vs freeequivalent combination therapies for hypertension: a meta-analysis of health care costs and adherence. J Clin Hypertens (Greenwich). 2011; 13:898–909. DOI: 10.1111/j.1751-7176.2011.00550.x. PMID: 22142349.
Article16. Sakima A, Ohshiro K, Nakada S, Yamazato M, Kohagura K, Nakamoto M, et al. Switching therapy from variable-dose multiple pill to fixed-dose single-pill combinations of angiotensin II receptor blockers and thiazides for hypertension. Clin Exp Hypertens. 2011; 33:309–315. DOI: 10.3109/10641963.2010.549260. PMID: 21649528.
Article17. EMA Guideline on the Investigation of Bioequivalence. London: European Medicines Agency (EMA);2010.18. FDA Bioequivalence Studies with Pharmacokinetic Endpoints for Drugs Submitted Under an ANDA. Guidance for Industry. 2013.19. MFDS Guidelines for Bioequivalence Studies for Drugs. MFDS (Ministry of Food and Drug Safety);2014.20. Van Peer A. Variability and impact on design of bioequivalence studies. Basic Clin Pharmacol Toxicol. 2010; 106:146–153. DOI: 10.1111/j.1742-7843.2009.00485.x. PMID: 20041877.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pharmacokinetic comparison between a fixed-dose combination of fimasartan/amlodipine/ hydrochlorothiazide 60/10/25 mg and a corresponding loose combination of fimasartan/amlodipine 60/25 mg and hydrochlorothiazide 25 mg in healthy subjects
- Pharmacokinetics and pharmacodynamics of a fixed-dose combination of gemigliptin/metformin sustained release 25/500 mg compared to the loose combination in healthy male subjects
- Pharmacokinetics and bioequivalence of fixed-dose combination of candesartan cilexetil/amlodipine besylate (16/10 mg) versus coadministration of individual formulations in healthy subjects
- Bioequivalence of HCP1104, a New Fixed Dose Combination Drug and Co-administration of Eperisone 50 mg and Aceclofenac 100 mg: A Partial Replicated Crossover Study Design to Estimate the Pharmacokinetics of Highly Variable Drugs
- Pharmacokinetic Equivalence of the High Dose Strength Fixed-Dose Combination Tablet of Gemigliptin/Metformin Sustained Release (SR) and Individual Component Gemigliptin and Metformin XR Tablets in Healthy Subjects