J Korean Soc Clin Pharmacol Ther.
2013 Dec;21(2):120-129. 10.12793/jkscpt.2013.21.2.120.
Bioequivalence of HCP1104, a New Fixed Dose Combination Drug and Co-administration of Eperisone 50 mg and Aceclofenac 100 mg: A Partial Replicated Crossover Study Design to Estimate the Pharmacokinetics of Highly Variable Drugs
- Affiliations
-
- 1Department of Clinical Pharmacology and Therapeutics, Asan Medical Center, Seoul, Korea. ksbae13@gmail.com
- 2Department of Clinical Pharmacology and Therapeutics, University of Ulsan College of Medicine, Seoul, Korea.
- 3Department of Pharmacology, Inje University College of Medicine, Busan, Korea.
- 4Department of Clinical Pharmacology, Inje University Busan Paik Hospital, Busan, Korea.
- KMID: 2203225
- DOI: http://doi.org/10.12793/jkscpt.2013.21.2.120
Abstract
- BACKGROUND
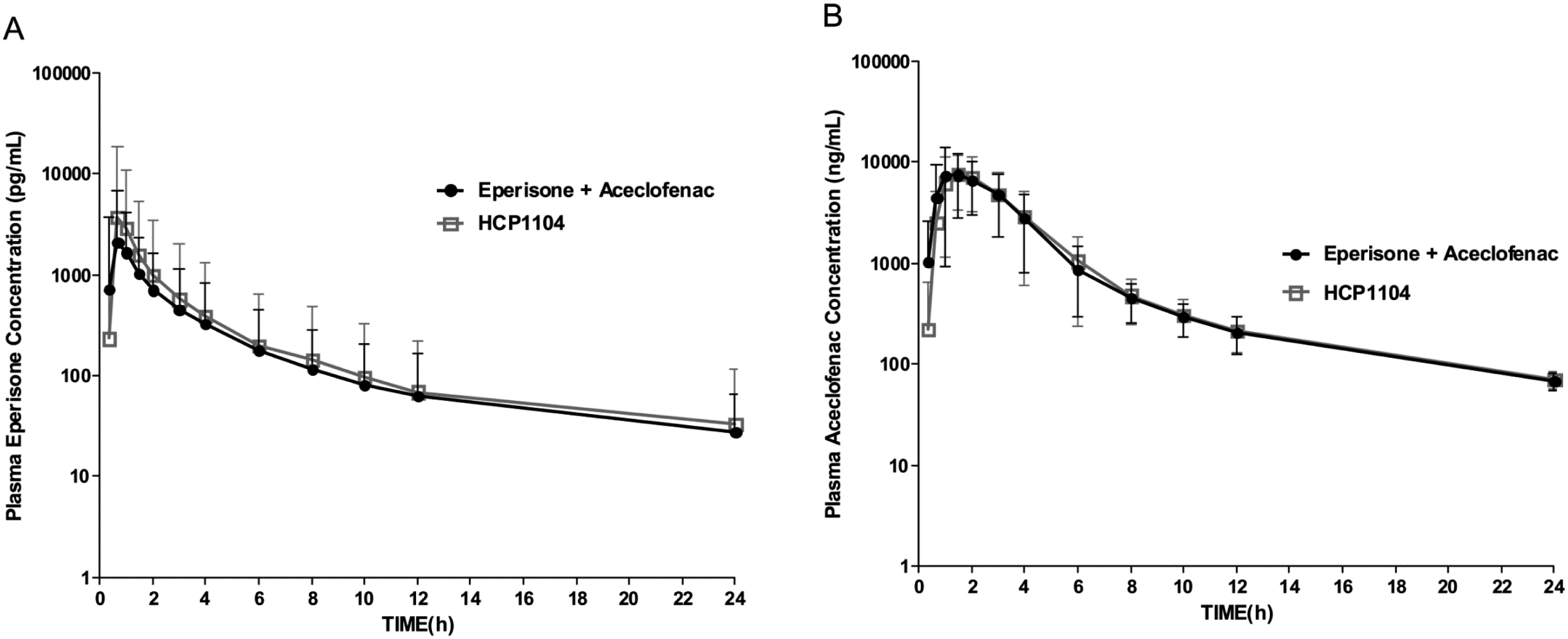
This clinical study was conducted to compare pharmacokinetics of eperisone and aceclofenac of HCP1104, a new fixed dose combination drug with those in co-administration of eperisone 50 mg and aceclofenac 100 mg. The study used a partial replicated study design to characterize intra-subject variability of eperisone when co-administrated with aceclofenac.
METHODS
A partial replicated crossover design was employed in 30 subjects. Each subject received a single dose of co-administration of eperisone 50 mg and aceclofenac 100 mg on two occasions and a single dose of 1 capsule of HCP1104. Blood samples were obtained for 24 hrs after dosing, and plasma was assayed for eperisone and aceclofenac by Liquid chromatography-electrospray ionization-mass spectrometry.
RESULTS
Using an average bioequivalence criterion, the 90 % confidence limits for Ln-transformed Cmax and AUClast for aceclofenac fell wihin the acceptable range of 80 - 125 %. Point estimates of eperisone AUClast and Cmax were 1.0152 and 1.0490, respectively and the 90 % confidence interval for Cmax was 0.8499 - 1.3025. The within-subject coefficient of variation of Cmax for the reference was 50.198 %. Acceptance range for eperisone Cmax based on new bioequivalence guidance for highly variable drugs was extended to 0.6984 - 1.4319.
CONCLUSION
The extent of exposure and rate of absorption of both eperisone and aceclofenac with a single dose of HCP1104 capsule were equivalent to those with co-administration of a marketed eperisone 50 mg tablet and a marketed aceclofenac 100 mg tablet under fasting conditions in healthy adult males.
Keyword
MeSH Terms
Figure
Reference
-
1. Dooley M, Spencer CM, Dunn CJ. Aceclofenac: a reappraisal of its use in the management of pain and rheumatic disease. Drugs. 2001; 61(9):1351–1378.2. Brogden RN, Wiseman LR. Aceclofenac. A review of its pharmacodynamic properties and therapeutic potential in the treatment of rheumatic disorders and in pain management. Drugs. 1996; 52(1):113–124.3. Melilli B, Piazza C, Vitale DC, Marano MR, Pecori A, Mattana P, Volsi VL, Iuculano C, Cardi F, Drago F. Human pharmacokinetics of the muscle relaxant, eperisone hydrochloride by liquid chromatography-electrospray tandem mass spectrometry. Eur J Drug Metab Pharmacokinet. 2011; 36(2):71–78.
Article4. Sartini S, Guerra L. Open experience with a new myorelaxant agent for low back pain. Adv Ther. 2008; 25(10):1010–1018.
Article5. Myonal drug information. http://www.eisai.jp/medical/products/di/EPI/MYO_T-G_EPI.pdf. [Online] (last visited on Nov 15 2013).6. Tothfalusi L, Endrenyi L. Sample sizes for designing bioequivalence studies for highly variable drugs. J Pharm Pharm Sci. 2012; 15(1):73–84.
Article7. Kim MJ, Lim HS, Noh YH, Kim YH, Choi HY, Park KM, Kim SE, Bae KS. Pharmacokinetic interactions between eperisone hydrochloride and aceclofenac: a randomized, open-label, crossover study of healthy korean men. Clin Ther. 2013; 35(10):1528–1535.
Article8. World Medical Association. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Bull World Health Organ. 2001; 79(4):373–374.9. RBORT XP, CARRASCO E., GOMEZLECHEON M.J., CASTEL J.V.Metabolism of aceclofenac in humans. Drug metabolism and disposition. 1996; 24(8):834–841.10. Agency EM. Guideline on the Investigation of Bioequivalence. Committee for Medicinal Products for Human Use. CHMP;London: 2010.11. Davit BM, Conner DP, Fabian-Fritsch B, Haidar SH, Jiang X, Patel DT, Seo PR, Suh K, Thompson CL, Yu LX. Highly variable drugs: observations from bioequivalence data submitted to the FDA for new generic drug applications. AAPS J. 2008; 10(1):148–156.
Article12. Karalis V, Symillides M, Macheras P. Bioequivalence of highly variable drugs: a comparison of the newly proposed regulatory approaches by FDA and EMA. Pharm Res. 2012; 29(4):1066–1077.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pharmacokinetic comparison between fixed-dose combination of fimasartan/amlodipine 60/10 mg and the corresponding loose combination through partial replicated crossover study in healthy subjects
- Comparison of pharmacokinetics and safety of fixed-dose combination of SKI306X and aceclofenac versus separate tablets in healthy subjects
- Pharmacokinetics of fixed-dose combination of rosuvastatin 20 mg and ezetimibe 10 mg compared to concurrent administration of individual tablets in healthy Korean subjects
- Pharmacokinetics and bioequivalence of fixed-dose combination of candesartan cilexetil/amlodipine besylate (16/10 mg) versus coadministration of individual formulations in healthy subjects
- Pharmacokinetic comparison between a fixed-dose combination of fimasartan/amlodipine/ hydrochlorothiazide 60/10/25 mg and a corresponding loose combination of fimasartan/amlodipine 60/25 mg and hydrochlorothiazide 25 mg in healthy subjects