Transl Clin Pharmacol.
2019 Dec;27(4):119-122. 10.12793/tcp.2019.27.4.119.
Elucidation of the pathophysiology of intradialytic muscle cramps: pharmacokinetics applied to translational research
- Affiliations
-
- 1Department of Pharmacology, Feinberg School of Medicine, Northwestern University, Chicago, Illinois, USA. art_atkinson@msn.com
- KMID: 2467964
- DOI: http://doi.org/10.12793/tcp.2019.27.4.119
Abstract
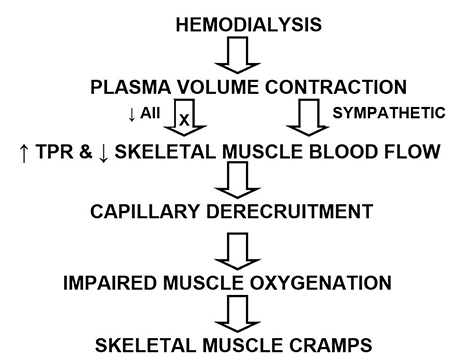
- In the conventional concept of translational research, investigations flow from the laboratory bench to the bedside. However, clinical research can also serve as the starting point for subsequent laboratory investigations that then lead back to the bedside. This article chronicles the evolution of a series of studies in which a detailed analysis of pharmacokinetics in hemodialysis patients revealed new physiological insight that, through a systems approach incorporating kinetic, physicochemical, physiologic, and clinical trial results, led to an elucidation of the pathophysiology of intradialytic skeletal muscle cramps. Based on this understanding, a therapeutic path forward is proposed.
Keyword
MeSH Terms
Figure
Reference
-
1. Elson J, Strong JM, Lee WK, Atkinson AJ Jr. The antiarrhythmic potency of N-acetylprocainamide. Clin Pharmacol Ther. 1975; 17:134–140.2. Strong JM, Dutcher JS, Lee WK, Atkinson AJ Jr. Pharmacokinetics in man of the N-acetylated metabolite of procainamide. J Pharmacokinet Biopharm. 1975; 3:223–235.3. Lee WK, Strong JM, Kehoe RF, Dutcher JS, Atkinson AJ Jr. Antiarrhythmic efficacy of N-acetylprocainamide in patients with premature ventricular contractions. Clin Pharmacol Ther. 1976; 19:508–514.
Article4. Stec GP, Atkinson AJ Jr, Nevin MJ, Thenot JP, Ruo TI, Gibson TP, et al. N-acetylprocainamide pharmacokinetics in functionally anephric patients before and after perturbation by hemodialysis. Clin Pharmacol Ther. 1979; 26:618–628.
Article5. Bowsher DH, Avram MJ, Frederiksen MC, Asada A, Atkinson AJ Jr. Urea distribution kinetics analyzed by simultaneous injection of urea and inulin. Demonstration that transcapillary exchange is rate limiting. J Pharmacol Exp Ther. 1984; 230:269–274.6. Odeh YK, Wang Z, Ruo TI, Wang T, Frederiksen MC, Pospisil PA, et al. Simultaneous analysis of inulin and 13N2-urea kinetics in humans. Clin Pharmacol Ther. 1993; 53:419–425.
Article7. Yim DS. “Physiological spaces and multicompartmental pharmacokinetic models”: fundamentals that pharmacokinetics textbooks do not tell you. Transl Clin Pharmacol. 2015; 23:35–37.
Article8. Renkin EM. Effects of blood flow on diffusion kinetics in isolated perfused hindlegs of cats: a double circulation hypothesis. Am J Physiol. 1955; 183:125–136.9. Paaske WP, Sejrsen P. Transcapillary exchange of 14C-inulin by free diffusion in channels of fused vesicles. Acta Physiol Scand. 1977; 100:437–445.
Article10. Belknap SM, Nelson JE, Ruo TI, Frederiksen MC, Worwag EM, Shin SG, et al. Theophylline distribution kinetics analyzed by reference to simultaneously injected urea and inulin. J Pharmacol Exp Ther. 1987; 243:963–969.11. Sedek GS, Ruo TI, Frederiksen MC, Frederiksen JW, Shih SR, Atkinson AJ Jr. Splanchnic tissues are a major part of the rapid distribution spaces of inulin, urea, and theophylline. J Pharmacol Exp Ther. 1989; 251:1026–1031.12. Bowsher DJ, Krejcie TC, Avram MJ, Chow MJ, del Greco F, Atkinson AJ Jr. Reduction in slow intercompartmental clearance of urea during dialysis. J Lab Clin Med. 1985; 105:489–497.13. Atkinson AJ Jr. The 1989 Harry Gold Award lecture: a pharmacokinetic odyssey. Pharmacologist. 1989; 31:229–234.14. Friesenecker B, Tsai AG, Dünser MW, Mayr AJ, Martini J, Knotzer H, et al. Oxygen distribution in microcirculation after arginine vasopressin-induced arteriolar vasoconstriction. Am J Physiol Heart Circ Physiol. 2004; H1792–H1800.
Article15. Järhult J. Comparative effects of angiotensin and noradrenaline on resistance, capacitance, and precapillary sphincter vessels in cat skeletal muscle. Acta Physiol Scand. 1971; 81:315–324.
Article16. Kaplan B, Wang T, Rammohan M, del Greco F, Molteni A, Atkinson AJ Jr. Response to head-up tilt in cramping and noncramping hemodialysis patients. Int J Clin Pharmacol Ther Toxicol. 1992; 30:173–180.17. Naik RB, Mathias CJ, Wilson CA, Reid JL, Warren DJ. Cardiovascular and autonomic reflexes in haemodialysis patients. Clin Sci (Lond). 1981; 60:165–170.
Article18. Kaji DM, Ackad A, Nottage WG, Stein RM. Prevention of muscle cramps in haemodialysis patients by quinine sulfate. Lancet. 1976; 2:66–67.19. Mecca TE, Elam JT, Nash CB, Caldwell RW. α-Adrenergic blocking properties of quinine HCl. Eur J Pharmacol. 1980; 63:159–166.
Article20. Sidhom OA, Odeh YK, Krumlovsky FA, Budris WA, Wang Z, Pospisil PA, et al. Low-dose prazosin in patients with muscle cramps during hemodialysis. Clin Pharmacol Ther. 1994; 56:445–451.
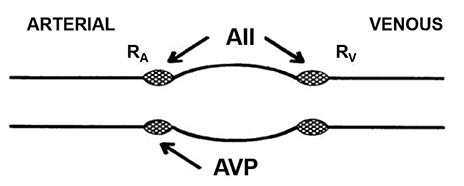
Article21. Piergies AA, Atkinson AJ Jr, Hubler GL, del Greco F. Activation of the renin-angiotensin system does not cause skeletal muscle cramps. Int J Clin Pharmacol Ther Toxicol. 1990; 28:405–409.22. Wakefield BJ, Busse LW, Khanna AK. Angiotensin II in vasodilatory shock. Crit Care Clin. 2019; 35:229–245. DOI: 10.1016/j.ccc.2018.11.003.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Muscle Cramps in Old Adults: Clinical Features and Pathophysiology
- Efficacy of Phenytoin for Nocturnal Muscle Cramps: A Preliminary Study
- Muscle Cramps in Liver Cirrhosis : Clinical Features and Related Factors
- Muscle Cramps in Patients with Chronic Liver Disease
- Effectiveness of 4-Week Oral Taurine Treatment for Muscle Cramps in Patients with Liver Cirrhosis: A Single-Arm Pilot Study