J Clin Neurol.
2020 Jan;16(1):19-28. 10.3988/jcn.2020.16.1.19.
Association between Retinal Vascular Geometric Changes and Cognitive Impairment: A Systematic Review and Meta-Analysis
- Affiliations
-
- 1Department of Medical Informatics, Medical School of Nantong University, Nantong, China.
- 2Department of Ophthalmology, Affiliated Hospital of Nantong University, Nantong, China. sangam@ntu.edu.cn
- KMID: 2467780
- DOI: http://doi.org/10.3988/jcn.2020.16.1.19
Abstract
- BACKGROUND AND PURPOSE
Previous studies have explored the association between retinal vascular changes and cognitive impairment. The retinal vasculature shares some characteristics with the cerebral vasculature, and quantitative changes in it could indicate cognitive impairment. Hence, a comprehensive meta-analysis was performed to clarify the potential relationship between retinal vascular geometric changes and cognitive impairment.
METHODS
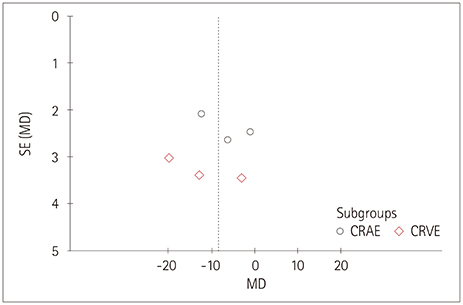
Relevant databases were scrupulously and systematically searched for retinal vascular geometric changes including caliber, tortuosity, and fractal dimension (FD), and for cognitive impairment. The Newcastle-Ottawa Scale was used to evaluate the methodological quality of included studies. RevMan was used to perform the meta-analysis and detect publication bias. Sensitivity analyses were also performed.
RESULTS
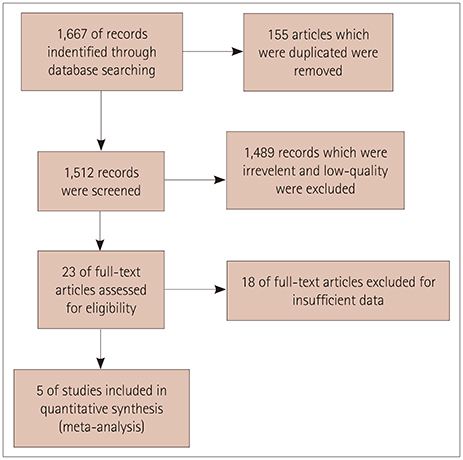
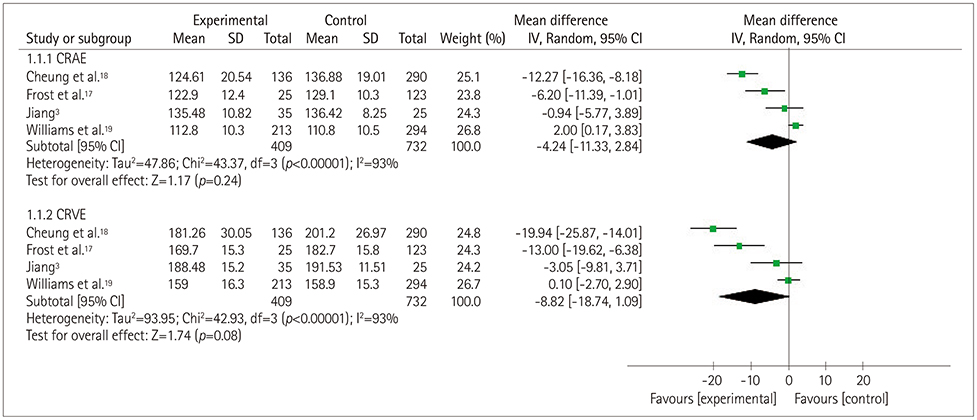
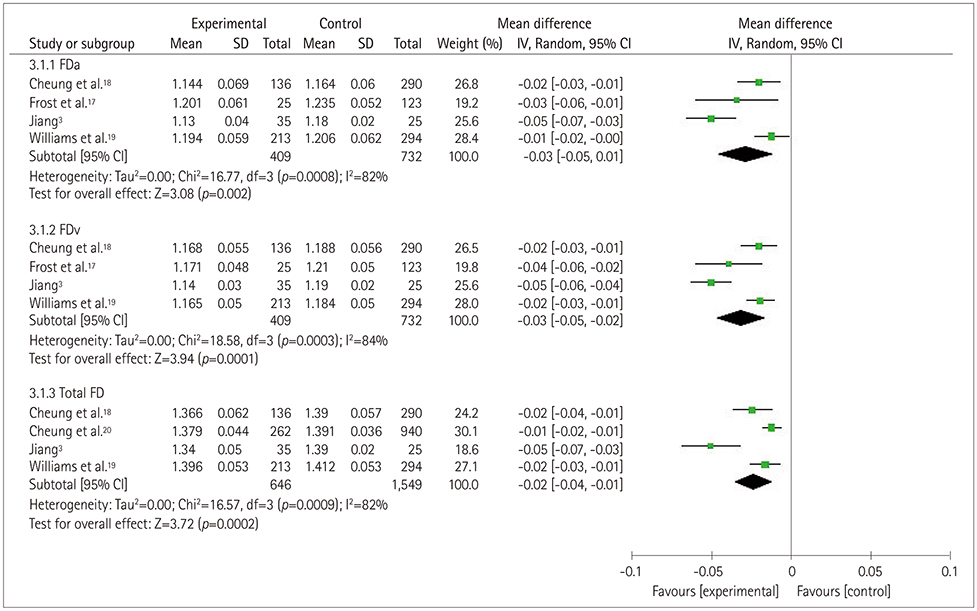
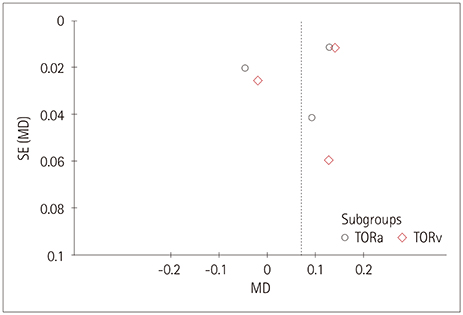
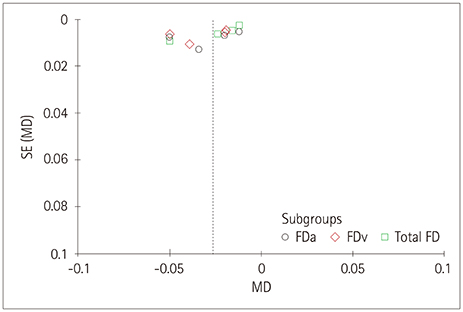
Five studies that involved 2,343 subjects were finally included in the meta-analysis. The results showed that there was no significant association between central retinal artery equivalents (Z=1.17) or central retinal venular equivalents (Z=1.74) and cognitive impairment (both p>0.05). Similarly, no significant difference was detected in retinal arteriolar tortuosity (Z=0.91) and venular tortuosity (Z=1.31) (both p>0.05). However, the retinal arteriolar FD (mean difference: −0.03, 95% CI: −0.05, −0.01) and venular FD (mean difference: −0.03, 95% CI: −0.05, −0.02) were associated with cognitive impairment.
CONCLUSIONS
A smaller retinal microvascular FD might be associated with cognitive impairment. Further large-sample and well-controlled original studies are required to confirm the present findings.
Keyword
MeSH Terms
Figure
Reference
-
1. Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011; 7:137–152.
Article2. Petersen RC, Roberts RO, Knopman DS, Geda YE, Cha RH, Pankratz VS, et al. Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology. 2010; 75:889–897.
Article3. Jiang YS. Retinal microvascular pathology and vascular risk factors in cognitive impairment: a cross-sectional study [dissertation]. Wuhan: Wuhan University;2015. 1–83.4. James BD, Leurgans SE, Hebert LE, Scherr PA, Yaffe K, Bennett DA. Contribution of Alzheimer disease to mortality in the United States. Neurology. 2014; 82:1045–1050.
Article5. Lesage SR, Mosley TH, Wong TY, Szklo M, Knopman D, Catellier DJ, et al. Retinal microvascular abnormalities and cognitive decline: the ARIC 14-year follow-up study. Neurology. 2009; 73:862–868.
Article6. Kim DH, Chaves PH, Newman AB, Klein R, Sarnak MJ, Newton E, et al. Retinal microvascular signs and disability in the Cardiovascular Health Study. Arch Ophthalmol. 2012; 130:350–356.
Article7. Ikram MK, De Jong FJ, Van Dijk EJ, Prins ND, Hofman A, Breteler MM, et al. Retinal vessel diameters and cerebral small vessel disease: the Rotterdam Scan Study. Brain. 2006; 129:182–188.
Article8. Schrijvers EM, Buitendijk GH, Ikram MK, Koudstaal PJ, Hofman A, Vingerling JR, et al. Retinopathy and risk of dementia: the Rotterdam Study. Neurology. 2012; 79:365–370.
Article9. Kawasaki R, Cheung N, Mosley T, Islam AF, Sharrett AR, Klein R, et al. Retinal microvascular signs and 10-year risk of cerebral atrophy: the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2010; 41:1826–1828.
Article10. Cheung N, Mosley T, Islam A, Kawasaki R, Sharrett AR, Klein R, et al. Retinal microvascular abnormalities and subclinical magnetic resonance imaging brain infarct: a prospective study. Brain. 2010; 133:1987–1993.
Article11. Dashtbozorg B, Mendonça AM, Penas S, Campilho A. RetinaCAD, a system for the assessment of retinal vascular changes. Conf Proc IEEE Eng Med Biol Soc. 2014; 2014:6328–6331.
Article12. Ponto KA, Werner DJ, Wiedemer L, Laubert-Reh D, Schuster AK, Nickels S, et al. Retinal vessel metrics: normative data and their use in systemic hypertension: results from the Gutenberg Health Study. J Hypertens. 2017; 35:1635–1645.13. De Jong FJ, Schrijvers EM, Ikram MK, Koudstaal PJ, De Jong PT, Hofman A, et al. Retinal vascular caliber and risk of dementia: the Rotterdam study. Neurology. 2011; 76:816–821.
Article14. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984; 34:939–944.
Article15. Ridha B, Rossor M. The mini mental state examination. Pract Neurol. 2005; 5:298–303.
Article16. Wells G. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Appl Eng Agric. 2000; 18:727–734.17. Frost S, Kanagasingam Y, Sohrabi H, Vignarajan J, Bourgeat P, Salvado O, et al. Retinal vascular biomarkers for early detection and monitoring of Alzheimer's disease. Transl Psychiatry. 2013; 3:e233.
Article18. Cheung CY, Ong YT, Ikram MK, Ong SY, Li X, Hilal S, et al. Microvascular network alterations in the retina of patients with Alzheimer's disease. Alzheimers Dement. 2014; 10:135–142.
Article19. Williams MA, McGowan AJ, Cardwell CR, Cheung CY, Craig D, Passmore P, et al. Retinal microvascular network attenuation in Alzheimer's disease. Alzheimers Dement (Amst). 2015; 1:229–235.
Article20. Cheung CY, Ong S, Ikram MK, Ong YT, Chen CP, Venketasubramanian N, et al. Retinal vascular fractal dimension is associated with cognitive dysfunction. J Stroke Cerebrovasc Dis. 2014; 23:43–50.
Article21. Cheung CY, Hsu W, Lee ML, Wang JJ, Mitchell P, Lau QP, et al. A new method to measure peripheral retinal vascular caliber over an extended area. Microcirculation. 2010; 17:495–503.22. Knudtson MD, Lee KE, Hubbard LD, Wong TY, Klein R, Klein BE. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res. 2003; 27:143–149.
Article23. Hart WE, Goldbaum M, Côté B, Kube P, Nelson MR. Measurement and classification of retinal vascular tortuosity. Int J Med Inform. 1999; 53:239–252.
Article24. Liew G, Wang JJ, Cheung N, Zhang YP, Hsu W, Lee ML, et al. The retinal vasculature as a fractal: methodology, reliability, and relationship to blood pressure. Ophthalmology. 2008; 115:1951–1956.
Article25. Heringa SM, Bouvy WH, Van den Berg E, Moll AC, Kappelle LJ, Biessels GJ. Associations between retinal microvascular changes and dementia, cognitive functioning, and brain imaging abnormalities: a systematic review. J Cereb Blood Flow Metab. 2013; 33:983–995.
Article26. Ding J, Patton N, Deary IJ, Strachan MW, Fowkes FG, Mitchell RJ, et al. Retinal microvascular abnormalities and cognitive dysfunction: a systematic review. Br J Ophthalmol. 2008; 92:1017–1025.
Article27. Gatto NM, Varma R, Torres M, Wong TY, Johnson PL, Segal-Gidan F, et al. Retinal microvascular abnormalities and cognitive function in Latino adults in Los Angeles. Ophthalmic Epidemiol. 2012; 19:127–136.
Article28. Jinnouchi H, Kitamura A, Yamagishi K, Kiyama M, Imano H, Okada T, et al. Retinal vascular changes and prospective risk of disabling dementia: the circulatory risk in communities study (CIRCS). J Atheroscler Thromb. 2017; 24:687–695.
Article29. Jiang H, Wei Y, Shi Y, Wright CB, Sun X, Gregori G, et al. Altered macular microvasculature in mild cognitive impairment and Alzheimer disease. J Neuroophthalmol. 2018; 38:292–298.
Article30. Ong YT, Hilal S, Cheung CY, Xu X, Chen C, Venketasubramanian N, et al. Retinal vascular fractals and cognitive impairment. Dement Geriatr Cogn Dis Extra. 2014; 4:305–313.
Article31. Mroczkowska S, Benavente-Pérez A, Patel S, Qin L, Bentham P, Gherghel D. Retinal vascular dysfunction relates to cognitive impairment in Alzheimer disease. Alzheimer Dis Assoc Disord. 2014; 28:366–367.
Article32. Sun X, He G, Qing H, Zhou W, Dobie F, Cai F, et al. Hypoxia facilitates Alzheimer's disease pathogenesis by up-regulating BACE1 gene expression. Proc Natl Acad Sci U S A. 2006; 103:18727–18732.
Article33. Koike MA, Garcia FG, Kitazawa M, Green KN, Laferla FM. Long term changes in phospho-APP and tau aggregation in the 3xTg-AD mice following cerebral ischemia. Neurosci Lett. 2011; 495:55–59.
Article34. Patton N, Aslam T, Macgillivray T, Pattie A, Deary IJ, Dhillon B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: a rationale based on homology between cerebral and retinal microvasculatures. J Anat. 2005; 206:319–348.
Article35. Murray CD. The physiological principle of minimum work I. The vascular system and the cost of blood volume. Proc Natl Acad Sci U S A. 1926; 12:207–214.
Article36. Xu X, Jerskey BA, Cote DM, Walsh EG, Hassenstab JJ, Ladino ME, et al. Cerebrovascular perfusion among older adults is moderated by strength training and gender. Neurosci Lett. 2014; 560:26–30.
Article37. Wang YX, Fitch RM. Vascular stiffness: measurements, mechanisms and implications. Curr Vasc Pharmacol. 2004; 2:379–384.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correlates of Cognitive Impairment of Rheumatic Disease: Systematic Review and Meta-analysis
- Effects of Cognitive-based Interventions of Older Adults with Mild Cognitive Impairment: A Systematic Review and Meta-analysis
- Effects of Mobile Health Applications in Older Adults with Dementia or Mild Cognitive Impairment: A Systematic Review and Meta-Analysis
- Protective Role of 360° Laser Retinopexy in Patients with Rhegmatogenous Retinal Detachment: a Systematic Review and Meta-analysis
- Resting Heart Rate and Cognitive Decline: A Meta-Analysis of Prospective Cohort Studies