J Clin Neurol.
2019 Oct;15(4):454-460. 10.3988/jcn.2019.15.4.454.
High Seroprevalence and Index of Anti-John-Cunningham Virus Antibodies in Korean Patients with Multiple Sclerosis
- Affiliations
-
- 1Department of Neurology, Research Institute and Hospital of National Cancer Center, Goyang, Korea. hojinkim@ncc.re.kr
- KMID: 2467753
- DOI: http://doi.org/10.3988/jcn.2019.15.4.454
Abstract
- BACKGROUND AND PURPOSE
The anti-John-Cunningham virus (JCV)-antibody serostatus and index are used in the risk stratification of progressive multifocal leukoencephalopathy (PML) in multiple sclerosis (MS) patients treated with natalizumab. However, little information on these parameters is available for Asian countries. The purpose of this study was to determine the rate of seropositivity, index, and longitudinal index evolution in Korean patients with MS.
METHODS
The antibody seroprevalence was analyzed in 355 samples from 187 patients with clinically isolated syndrome or MS using a second-generation, two-step, enzyme-linked immunosorbent assay. A 4-year longitudinal evaluation was applied to 66 patients.
RESULTS
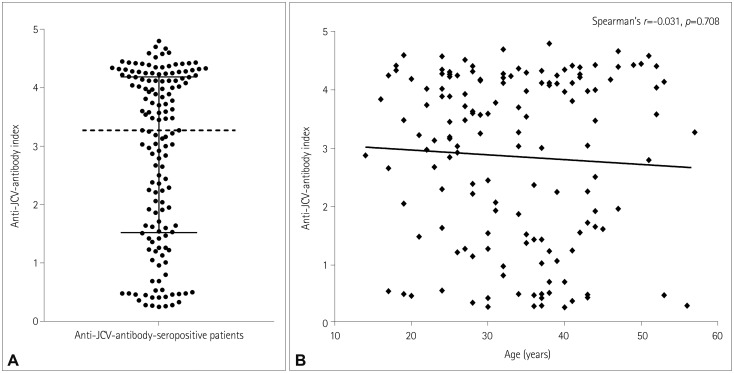
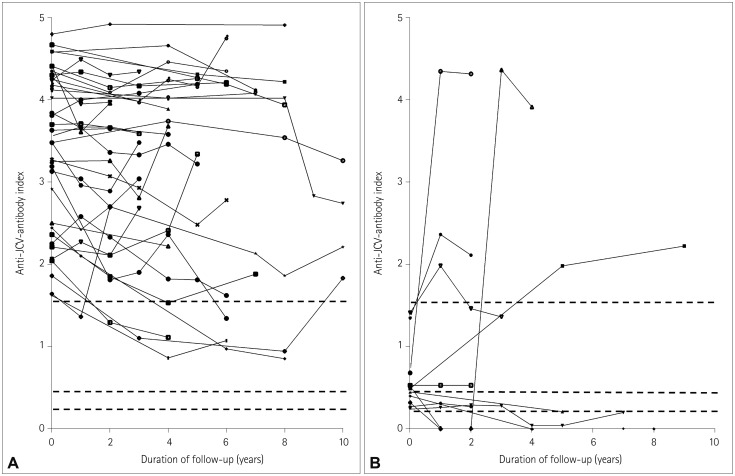
The overall antibody seroprevalence was 80% (n=149). Among antibody-positive patients, the index had a median value of 3.27 (interquartile range, 1.52-4.18), with 77% (n=114) and 56% (n=83) of patients having indices >1.5 and >3.0, respectively. The serostatus of 59 (89%) of the 66 patients did not change during the longitudinal analysis, while 3 (6%) of the 53 patients who were initially seropositive reverted to seronegativity, and 2 (15%) of the 13 patients who were initially seronegative converted to seropositivity. All patients with a baseline index >0.9 maintained seropositivity, and 92% of patients with a baseline index >1.5 maintained this index over 4 years. No patients developed PML (median disease duration, 8 years).
CONCLUSIONS
The seroprevalence and index of anti-JCV antibodies in Korean patients with MS may be higher than those in Western countries.
MeSH Terms
Figure
Reference
-
1. Bloomgren G, Richman S, Hotermans C, Subramanyam M, Goelz S, Natarajan A, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med. 2012; 366:1870–1880. PMID: 22591293.
Article2. Plavina T, Subramanyam M, Bloomgren G, Richman S, Pace A, Lee S, et al. Anti-JC virus antibody levels in serum or plasma further define risk of natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol. 2014; 76:802–812. PMID: 25273271.
Article3. Ho PR, Koendgen H, Campbell N, Haddock B, Richman S, Chang I. Risk of natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: a retrospective analysis of data from four clinical studies. Lancet Neurol. 2017; 16:925–933. PMID: 28969984.
Article4. Correia I, Jesus-Ribeiro J, Batista S, Martins AI, Nunes C, Macário MC, et al. Anti-JCV antibody serostatus and longitudinal evaluation in a Portuguese multiple sclerosis population. J Clin Neurosci. 2017; 45:257–260. PMID: 28844615.
Article5. Dominguez-Mozo MI, Rus M, Santiago JL, Izquierdo G, Casanova I, Galan V, et al. Study of the anti-JCV antibody levels in a Spanish multiple sclerosis cohort. Eur J Clin Invest. 2017; 47:158–166. PMID: 28036121.
Article6. Hegen H, Auer M, Bsteh G, Di Pauli F, Plavina T, Walde J, et al. Stability and predictive value of anti-JCV antibody index in multiple sclerosis: a 6-year longitudinal study. PLoS One. 2017; 12:e0174005. PMID: 28319193.
Article7. Raffel J, Gafson AR, Malik O, Nicholas R. Anti-JC virus antibody titres increase over time with natalizumab treatment. Mult Scler. 2015; 21:1833–1838. PMID: 26449743.
Article8. Schwab N, Schneider-Hohendorf T, Pignolet B, Breuer J, Gross CC, Göbel K, et al. Therapy with natalizumab is associated with high JCV seroconversion and rising JCV index values. Neurol Neuroimmunol Neuroinflamm. 2016; 3:e195. PMID: 26848486.
Article9. Lee P, Plavina T, Castro A, Berman M, Jaiswal D, Rivas S, et al. A second-generation ELISA (STRATIFY JCV™ DxSelect™) for detection of JC virus antibodies in human serum and plasma to support progressive multifocal leukoencephalopathy risk stratification. J Clin Virol. 2013; 57:141–146. PMID: 23465394.
Article10. Kim SH, Hyun JW, Jeong IH, Joung A, Yeon JL, Dehmel T, et al. Anti-JC virus antibodies in rituximab-treated patients with neuromyelitis optica spectrum disorder. J Neurol. 2015; 262:696–700. PMID: 25559683.
Article11. Lau A, Qiu W, Kermode A, Au C, Ng A, Wong A, et al. High prevalence and indexes of anti-John Cunningham virus antibodies in a cohort of Chinese patients with multiple sclerosis. Mult Scler J Exp Transl Clin. 2018; 4:2055217318788699. PMID: 30038791.
Article12. Aoyama S, Mori M, Uzawa A, Uchida T, Masuda H, Ohtani R, et al. Serum anti-John Cunningham virus antibody seroprevalence and index among Japanese patients with neuromyelitis optica spectrum disorders. Mult Scler. 2018; 10. 26. DOI: 10.1177/1352458518808473. [Epub].
Article13. Aoyama S, Mori M, Uzawa A, Uchida T, Masuda H, Ohtani R, et al. Serum anti-JCV antibody indexes in Japanese patients with multiple sclerosis: elevations along with fingolimod treatment duration. J Neurol. 2018; 265:1145–1150. PMID: 29532286.
Article14. Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005; 58:840–846. PMID: 16283615.
Article15. Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011; 69:292–302. PMID: 21387374.
Article16. Paz SPC, Branco L, Pereira MAC, Spessotto C, Fragoso YD. Systematic review of the published data on the worldwide prevalence of John Cunningham virus in patients with multiple sclerosis and neuromyelitis optica. Epidemiol Health. 2018; 40:e2018001. PMID: 29370683.
Article17. Kolasa M, Hagman S, Verkkoniemi-Ahola A, Airas L, Koivisto K, Elovaara I. Anti-JC virus seroprevalence in a Finnish MS cohort. Acta Neurol Scand. 2016; 133:391–397. PMID: 26347001.
Article18. Vennegoor A, Van Rossum JA, Polman CH, Wattjes MP, Killestein J. Longitudinal JCV serology in multiple sclerosis patients preceding natalizumab-associated progressive multifocal leukoencephalopathy. Mult Scler. 2015; 21:1600–1603. PMID: 25662344.
Article19. Kitamura T, Kunitake T, Guo J, Tominaga T, Kawabe K, Yogo Y. Transmission of the human polyomavirus JC virus occurs both within the family and outside the family. J Clin Microbiol. 1994; 32:2359–2363. PMID: 7814466.
Article20. Kunitake T, Kitamura T, Guo J, Taguchi F, Kawabe K, Yogo Y. Parentto-child transmission is relatively common in the spread of the human polyomavirus JC virus. J Clin Microbiol. 1995; 33:1448–1451. PMID: 7650165.
Article21. Javed A, Reder AT. Rising JCV-Ab index during natalizumab therapy for MS: Inauspicious for a highly efficacious drug. Neurol Neuroimmunol Neuroinflamm. 2016; 3:e199. PMID: 26848488.22. Agostini HT, Ryschkewitsch CF, Stoner GL. Genotype profile of human polyomavirus JC excreted in urine of immunocompetent individuals. J Clin Microbiol. 1996; 34:159–164. PMID: 8748293.
Article23. Varella RB, Zalona ACJ, Diaz NC, Zalis MG, Santoro-Lopes G. BK polyomavirus genotypes Ia and Ib1 exhibit different biological properties in renal transplant recipients. Virus Res. 2018; 243:65–68. PMID: 29106916.
Article24. Sundqvist E, Buck D, Warnke C, Albrecht E, Gieger C, Khademi M, et al. JC polyomavirus infection is strongly controlled by human leucocyte antigen class II variants. PLoS Pathog. 2014; 10:e1004084. PMID: 24763718.
Article25. Oshima Y, Tanimoto T, Yuji K, Tojo A. Drug-associated progressive multifocal leukoencephalopathy in multiple sclerosis patients. Mult Scler. 2018; 7. 09. DOI: 10.1177/1352458518786075. [Epub].
Article26. Schwab N, Schneider-Hohendorf T, Melzer N, Cutter G, Wiendl H. Natalizumab-associated PML: challenges with incidence, resulting risk, and risk stratification. Neurology. 2017; 88:1197–1205. PMID: 28228564.27. Jelcic I, Jelcic I, Kempf C, Largey F, Planas R, Schippling S, et al. Mechanisms of immune escape in central nervous system infection with neurotropic JC virus variant. Ann Neurol. 2016; 79:404–418. PMID: 26874214.
Article28. Gorelik L, Reid C, Testa M, Brickelmaier M, Bossolasco S, Pazzi A, et al. Progressive multifocal leukoencephalopathy (PML) development is associated with mutations in JC virus capsid protein VP1 that change its receptor specificity. J Infect Dis. 2011; 204:103–114. PMID: 21628664.
Article29. Geoghegan EM, Pastrana DV, Schowalter RM, Ray U, Gao W, Ho M, et al. Infectious entry and neutralization of pathogenic JC polyomaviruses. Cell Rep. 2017; 21:1169–1179. PMID: 29091757.
Article30. Ray U, Cinque P, Gerevini S, Longo V, Lazzarin A, Schippling S, et al. JC polyomavirus mutants escape antibody-mediated neutralization. Sci Transl Med. 2015; 7:306ra151.
Article31. Vennegoor A, Van Rossum JA, Leurs C, Wattjes MP, Rispens T, Murk JL, et al. High cumulative JC virus seroconversion rate during long-term use of natalizumab. Eur J Neurol. 2016; 23:1079–1085. PMID: 27018481.
Article32. Schwab N, Schneider-Hohendorf T, Pignolet B, Spadaro M, Görlich D, Meinl I, et al. PML risk stratification using anti-JCV antibody index and L-selectin. Mult Scler. 2016; 22:1048–1060. PMID: 26432858.
Article33. Villar LM, Costa-Frossard L, Masterman T, Fernandez O, Montalban X, Casanova B, et al. Lipid-specific immunoglobulin M bands in cerebrospinal fluid are associated with a reduced risk of developing progressive multifocal leukoencephalopathy during treatment with natalizumab. Ann Neurol. 2015; 77:447–457. PMID: 25581547.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum to: High Seroprevalence and Index of Anti-John-Cunningham Virus Antibodies in Korean Patients with Multiple Sclerosis
- Systematic review of the published data on the worldwide prevalence of John Cunningham virus in patients with multiple sclerosis and neuromyelitis optica
- Epstein-Barr Virus Related Polymorphic Posttransplantation Lymphoproliferative Disease in a Patient with Latent Infection of JC Virus
- Development of Multiple Sclerosis in a Patient with Systemic Lupus Erythematosus
- Seroprevalence of Anti-hepatitis B Virus, Anti-hepatitis A Virus, and Anti-varicella Zoster Virus Antibodies in Nursing Students from 2009 to 2013