J Clin Neurol.
2019 Apr;15(2):211-220. 10.3988/jcn.2019.15.2.211.
Electroencephalographic Resting-State Functional Connectivity of Benign Epilepsy with Centrotemporal Spikes
- Affiliations
-
- 1Department of Electrical and Computer Engineering, Seoul National University, Seoul, Korea.
- 2Healthcare ICT Research Center, Seoul National University Bundang Hospital, Seongnam, Korea.
- 3Department of Pediatrics, Seoul National University Bundang Hospital, Seongnam, Korea. hunminkim@snubh.org
- 4Division of Medical Statistics, Medical Research Collaborating Center, Seoul National University Bundang Hospital, Seongnam, Korea.
- 5Pediatric Clinical Neuroscience Center, Seoul National University Children's Hospital, Seoul, Korea.
- 6Department of Pediatrics, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2467735
- DOI: http://doi.org/10.3988/jcn.2019.15.2.211
Abstract
- BACKGROUND AND PURPOSE
We aimed to reveal resting-state functional connectivity characteristics based on the spike-free waking electroencephalogram (EEG) of benign epilepsy with centrotemporal spikes (BECTS) patients, which usually appears normal in routine visual inspection.
METHODS
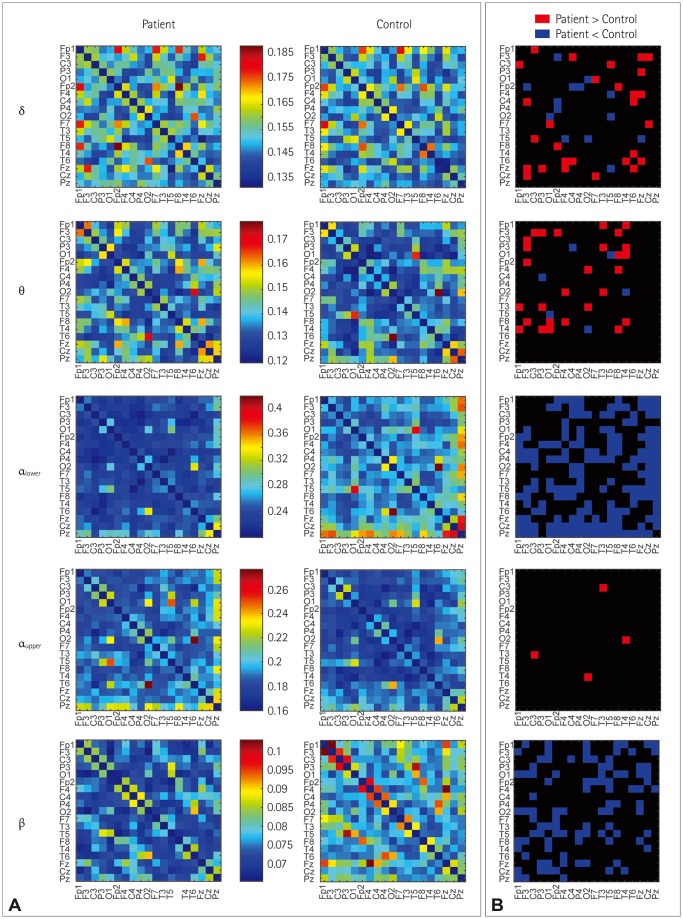
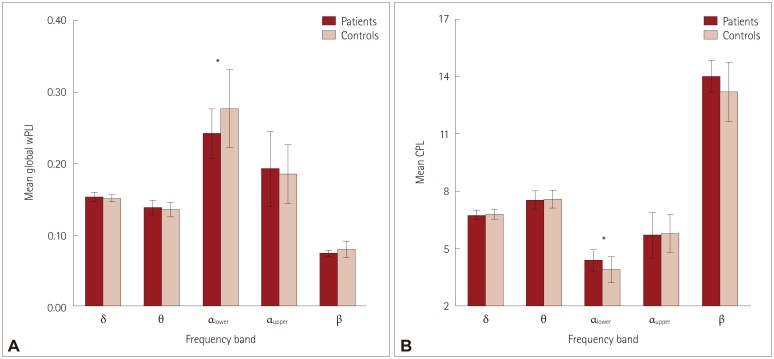
Thirty BECTS patients and 30 disease-free and age- and sex-matched controls were included. Eight-second EEG epochs without artifacts were sampled and then bandpass filtered into the delta, theta, lower alpha, upper alpha, and beta bands to construct the association matrix. The weighted phase lag index (wPLI) was used as an association measure for EEG signals. The band-specific connectivity, which was represented as a matrix of wPLI values of all edges, was compared for analyzing the connectivity itself. The global wPLI, characteristic path length (CPL), and mean clustering coefficient were compared.
RESULTS
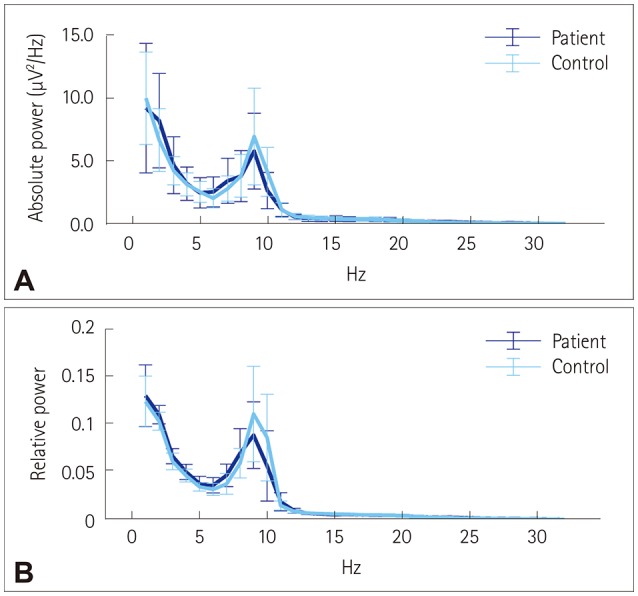
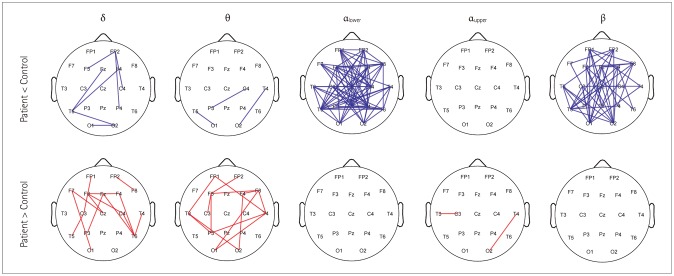
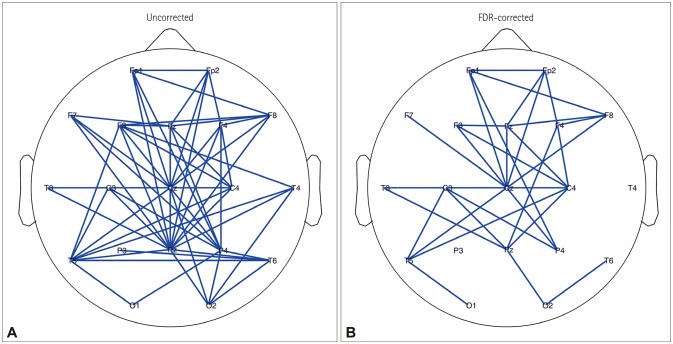
The resting-state functional connectivity itself and the network topology differed in the BECTS patients. For the lower-alpha-band and beta-band connectivity, edges that showed significant differences had consistently lower wPLI values compared to the disease-free controls. The global wPLI value was significantly lower for BECTS patients than for the controls in lower-alpha-band connectivity (mean±SD; 0.241±0.034 vs. 0.276±0.054, p=0.024), while the CPL was significantly longer for BECTS in the same frequency band (mean±SD; 4.379±0.574 vs. 3.904±0.695, p=0.04). The resting-state functional connectivity of BECTS showed decreased connectivity, integration, and efficiency compared to controls.
CONCLUSIONS
The connectivity differed significantly between BECTS patients and disease-free controls. In BECTS, global connectivity was significantly decreased and the resting-state functional connectivity showed lower efficiency in the lower alpha band.
Keyword
Figure
Reference
-
1. Wheless JW. Antiepileptic drug follow-up and withdrawal. In : Duchowny M, Cross JH, Arzimanoglou A, editors. Pediatric Epilepsy. New York: McGraw-Hill;2013. p. 386–387.2. Panayiotopoulos CP. The Epilepsies. Chipping Norton: Bladon Medical Publishing;2005.3. Lima EM, Rzezak P, Dos Santos B, Gentil L, Montenegro MA, Guerreiro MM, et al. The relevance of attention deficit hyperactivity disorder in self-limited childhood epilepsy with centrotemporal spikes. Epilepsy Behav. 2018; 82:164–169. PMID: 29649723.
Article4. Pellock JM, Nordli DR, Sankar R, Wheless J. Pellock’s Pediatric Epilepsy: Diagnosis and Therapy. 4th ed. New York: Demos Medical;2016. p. 359–361.5. Kim H, Kim SY, Lim BC, Hwang H, Chae JH, Choi J, et al. Spike persistence and normalization in benign epilepsy with centrotemporal spikes–Implications for management. Brain Dev. 2018; 40:693–698. PMID: 29754875.6. Stam CJ, Nolte G, Daffertshofer A. Phase lag index: assessment of functional connectivity from multi channel EEG and MEG with diminished bias from common sources. Hum Brain Mapp. 2007; 28:1178–1193. PMID: 17266107.
Article7. Vinck M, Oostenveld R, Van Wingerden M, Battaglia F, Pennartz CM. An improved index of phase-synchronization for electrophysiological data in the presence of volume-conduction, noise and sample-size bias. Neuroimage. 2011; 55:1548–1565. PMID: 21276857.
Article8. Adebimpe A, Aarabi A, Bourel-Ponchel E, Mahmoudzadeh M, Wallois F. Functional brain dysfunction in patients with benign childhood epilepsy as revealed by graph theory. PLoS One. 2015; 10:e0139228. PMID: 26431333.
Article9. Clemens B, Puskás S, Spisák T, Lajtos I, Opposits G, Besenyei M, et al. Increased resting-state EEG functional connectivity in benign childhood epilepsy with centro-temporal spikes. Seizure. 2016; 35:50–55. PMID: 26794010.
Article10. Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia. 1989; 30:389–399. PMID: 2502382.11. Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017; 58:512–521. PMID: 28276062.12. Fejerman N, Caraballo R, Tenembaum SN. Atypical evolutions of benign localization-related epilepsies in children: are they predictable? Epilepsia. 2000; 41:380–390. PMID: 10756401.
Article13. Kramer U. Atypical presentations of benign childhood epilepsy with centrotemporal spikes: a review. J Child Neurol. 2008; 23:785–790. PMID: 18658078.
Article14. Riviello JJ Jr, Nordli DR Jr, Niedermeyer E. Normal EEG and sleep: infants to adolescents. In : Donald LS, da Silva FH, editors. Niedermeyer’s Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. 6th ed. Philadelphia: Lippincott Williams & Wilkins;2011. p. 163–181.15. Van Diessen E, Numan T, Van Dellen E, Van der Kooi AW, Boersma M, Hofman D, et al. Opportunities and methodological challenges in EEG and MEG resting state functional brain network research. Clin Neurophysiol. 2015; 126:1468–1481. PMID: 25511636.
Article16. Sun J, Li Z, Tong S. Inferring functional neural connectivity with phase synchronization analysis: a review of methodology. Comput Math Methods Med. 2012; 2012:239210. PMID: 22577470.
Article17. Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010; 52:1059–1069. PMID: 19819337.
Article18. Zalesky A, Fornito A, Bullmore ET. Network-based statistic: identifying differences in brain networks. Neuroimage. 2010; 53:1197–1207. PMID: 20600983.
Article19. Adebimpe A, Aarabi A, Bourel-Ponchel E, Mahmoudzadeh M, Wallois F. EEG resting state functional connectivity analysis in children with benign epilepsy with centrotemporal spikes. Front Neurosci. 2016; 10:143. PMID: 27065797.
Article20. Douw L, De Groot M, Van Dellen E, Heimans JJ, Ronner HE, Stam CJ, et al. ‘Functional connectivity’ is a sensitive predictor of epilepsy diagnosis after the first seizure. PLoS One. 2010; 5:e10839. PMID: 20520774.
Article21. Chowdhury FA, Woldman W, FitzGerald TH, Elwes RD, Nashef L, Terry JR, et al. Revealing a brain network endophenotype in families with idiopathic generalised epilepsy. PLoS One. 2014; 9:e110136. PMID: 25302690.
Article22. Englot DJ, Hinkley LB, Kort NS, Imber BS, Mizuiri D, Honma SM, et al. Global and regional functional connectivity maps of neural oscillations in focal epilepsy. Brain. 2015; 138:2249–2262. PMID: 25981965.
Article23. Besseling RM, Jansen JF, Overvliet GM, Van der Kruijs SJ, Ebus SC, De Louw AJ, et al. Delayed convergence between brain network structure and function in rolandic epilepsy. Front Hum Neurosci. 2014; 8:704. PMID: 25249968.
Article24. Luo C, Qiu C, Guo Z, Fang J, Li Q, Lei X, et al. Disrupted functional brain connectivity in partial epilepsy: a resting-state fMRI study. PLoS One. 2011; 7:e28196. PMID: 22242146.
Article25. Wu Y, Ji GJ, Li K, Jin Z, Liu YL, Zeng YW, et al. Interhemispheric connectivity in drug-naive benign childhood epilepsy with centrotemporal spikes: combining function and diffusion MRI. Medicine (Baltimore). 2015; 94:e1550. PMID: 26376406.26. Righi M, Barcaro U, Starita A, Karakonstantaki E, Micheloyannis S. Detection of signs of brain dysfunction in epileptic children by recognition of transient changes in the correlation of seizure-free EEG. Brain Topogr. 2008; 21:43–51. PMID: 18566884.
Article27. Sargolzaei S, Cabrerizo M, Goryawala M, Eddin AS, Adjouadi M. Scalp EEG brain functional connectivity networks in pediatric epilepsy. Comput Biol Med. 2015; 56:158–166. PMID: 25464357.
Article28. Van Diessen E, Zweiphenning WJ, Jansen FE, Stam CJ, Braun KP, Otte WM. Brain network organization in focal epilepsy: a systematic review and meta-analysis. PLoS One. 2014; 9:e114606. PMID: 25493432.
Article29. Horstmann MT, Bialonski S, Noennig N, Mai H, Prusseit J, Wellmer J, et al. State dependent properties of epileptic brain networks: comparative graph-theoretical analyses of simultaneously recorded EEG and MEG. Clin Neurophysiol. 2010; 121:172–185. PMID: 20045375.
Article30. Bartolomei F, Bosma I, Klein M, Baayen JC, Reijneveld JC, Postma TJ, et al. Disturbed functional connectivity in brain tumour patients: evaluation by graph analysis of synchronization matrices. Clin Neurophysiol. 2006; 117:2039–2049. PMID: 16859985.
Article31. Van Dellen E, Douw L, Hillebrand A, Ris-Hilgersom IH, Schoonheim MM, Baayen JC, et al. MEG network differences between low- and high-grade glioma related to epilepsy and cognition. PLoS One. 2012; 7:e50122. PMID: 23166829.
Article32. Quraan MA, McCormick C, Cohn M, Valiante TA, McAndrews MP. Altered resting state brain dynamics in temporal lobe epilepsy can be observed in spectral power, functional connectivity and graph theory metrics. PLoS One. 2013; 8:e68609. PMID: 23922658.
Article33. Vlooswijk MC, Vaessen MJ, Jansen JF, De Krom MC, Majoie HJ, Hofman PA, et al. Loss of network efficiency associated with cognitive decline in chronic epilepsy. Neurology. 2011; 77:938–944. PMID: 21832213.
Article34. Ortiz E, Stingl K, Münssinger J, Braun C, Preissl H, Belardinelli P. Weighted phase lag index and graph analysis: preliminary investigation of functional connectivity during resting state in children. Comput Math Methods Med. 2012; 2012:186353. PMID: 23049617.
Article35. Hardmeier M, Hatz F, Bousleiman H, Schindler C, Stam CJ, Fuhr P. Reproducibility of functional connectivity and graph measures based on the phase lag index (PLI) and weighted phase lag index (wPLI) derived from high resolution EEG. PLoS One. 2014; 9:e108648. PMID: 25286380.
Article36. Zeng K, Wang Y, Ouyang G, Bian Z, Wang L, Li X. Complex network analysis of resting state EEG in amnestic mild cognitive impairment patients with type 2 diabetes. Front Comput Neurosci. 2015; 9:133. PMID: 26578946.
Article37. González JJ, Mañas S, De Vera L, Méndez LD, López S, Garrido JM, et al. Assessment of electroencephalographic functional connectivity in term and preterm neonates. Clin Neurophysiol. 2011; 122:696–702. PMID: 21074493.
Article38. Xing M, Tadayonnejad R, MacNamara A, Ajilore O, DiGangi J, Phan KL, et al. Resting-state theta band connectivity and graph analysis in generalized social anxiety disorder. Neuroimage Clin. 2016; 13:24–32. PMID: 27920976.
Article39. De Haan W, Pijnenburg YA, Strijers RL, Van der Made Y, Van der Flier WM, Scheltens P, et al. Functional neural network analysis in frontotemporal dementia and Alzheimer’s disease using EEG and graph theory. BMC Neurosci. 2009; 10:101. PMID: 19698093.
Article40. Shackman AJ, McMenamin BW, Maxwell JS, Greischar LL, Davidson RJ. Identifying robust and sensitive frequency bands for interrogating neural oscillations. Neuroimage. 2010; 51:1319–1333. PMID: 20304076.
Article41. Van Diessen E, Otte WM, Braun KP, Stam CJ, Jansen FE. Improved diagnosis in children with partial epilepsy using a multivariable prediction model based on EEG network characteristics. PLoS One. 2013; 8:e59764. PMID: 23565166.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Language-Related Functional Connectivity in Patients with Benign Childhood Epilepsy with Centrotemporal Spikes
- Children with Centrotemporal Spikes: Clinical and EEG Characteristics
- Resting-State Electroencephalography (EEG) Functional Connectivity Analysis
- Clinical Study of Benign Childhood Epilepsy with Centro-Temporal Spikes
- Cognitive and Behavioral Problems and the Effectiveness of Topiramate Once per Day in the Control of Benign Childhood Epilepsy with Centrotemporal Spikes