Development and Validation of a Deep Learning System for Segmentation of Abdominal Muscle and Fat on Computed Tomography
- Affiliations
-
- 1Department of Radiology and Research Institute of Radiology, Asan Image Metrics, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. medimash@gmail.com
- 2School of Computer Science and Engineering, Soongsil University, Seoul, Korea.
- 3Department of Nephrology, Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 4Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 5Department of Emergency Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 6Department of Radiology, Ajou University School of Medicine and Graduate School of Medicine, Ajou University Hospital, Suwon, Korea.
- 7Department of Radiology, Ulsan University Hospital, Ulsan, Korea.
- KMID: 2467045
- DOI: http://doi.org/10.3348/kjr.2019.0470
Abstract
OBJECTIVE
We aimed to develop and validate a deep learning system for fully automated segmentation of abdominal muscle and fat areas on computed tomography (CT) images.
MATERIALS AND METHODS
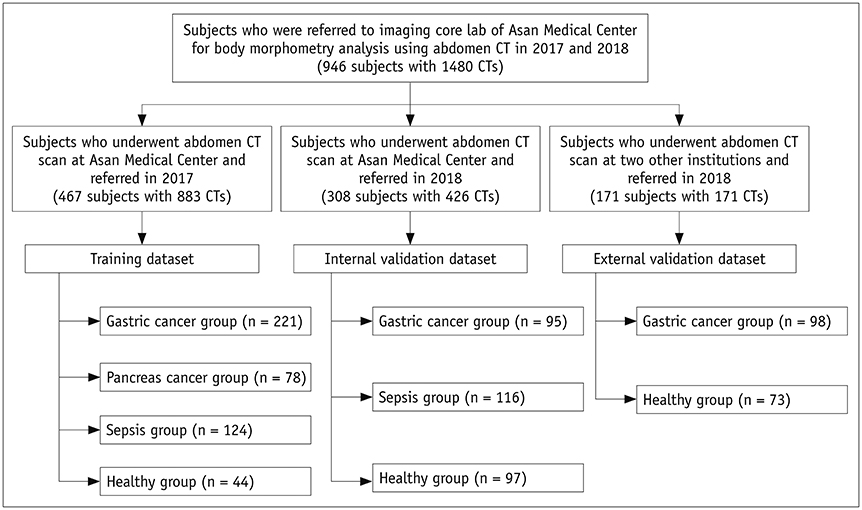
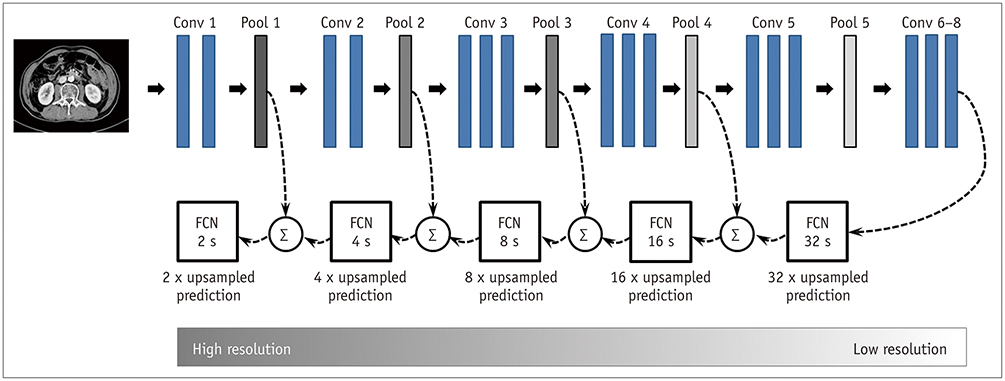
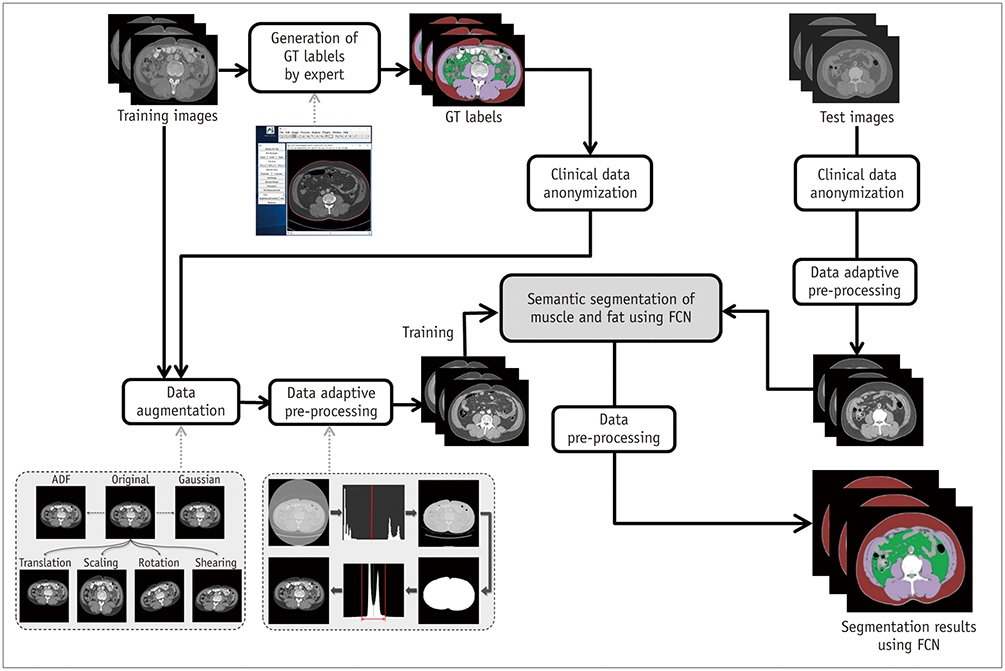
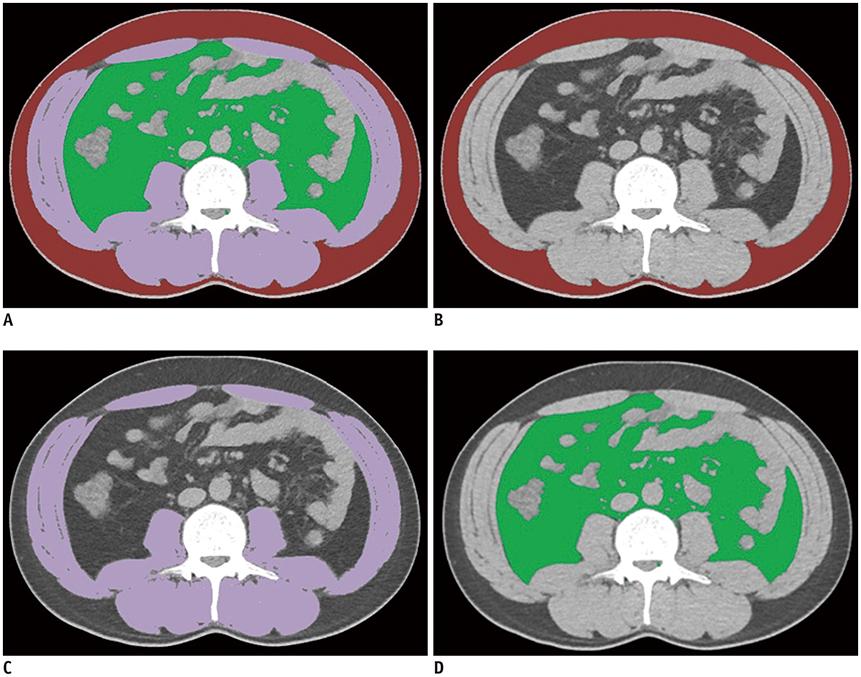
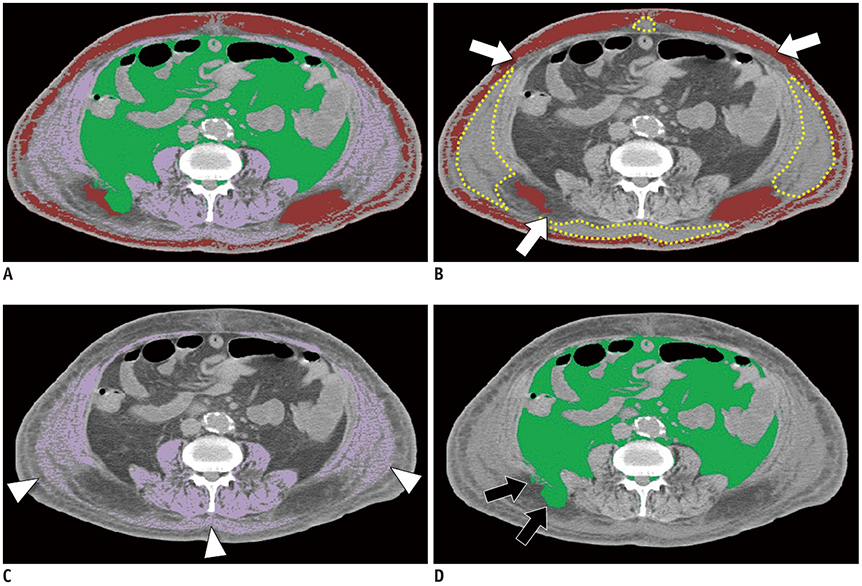
A fully convolutional network-based segmentation system was developed using a training dataset of 883 CT scans from 467 subjects. Axial CT images obtained at the inferior endplate level of the 3rd lumbar vertebra were used for the analysis. Manually drawn segmentation maps of the skeletal muscle, visceral fat, and subcutaneous fat were created to serve as ground truth data. The performance of the fully convolutional network-based segmentation system was evaluated using the Dice similarity coefficient and cross-sectional area error, for both a separate internal validation dataset (426 CT scans from 308 subjects) and an external validation dataset (171 CT scans from 171 subjects from two outside hospitals).
RESULTS
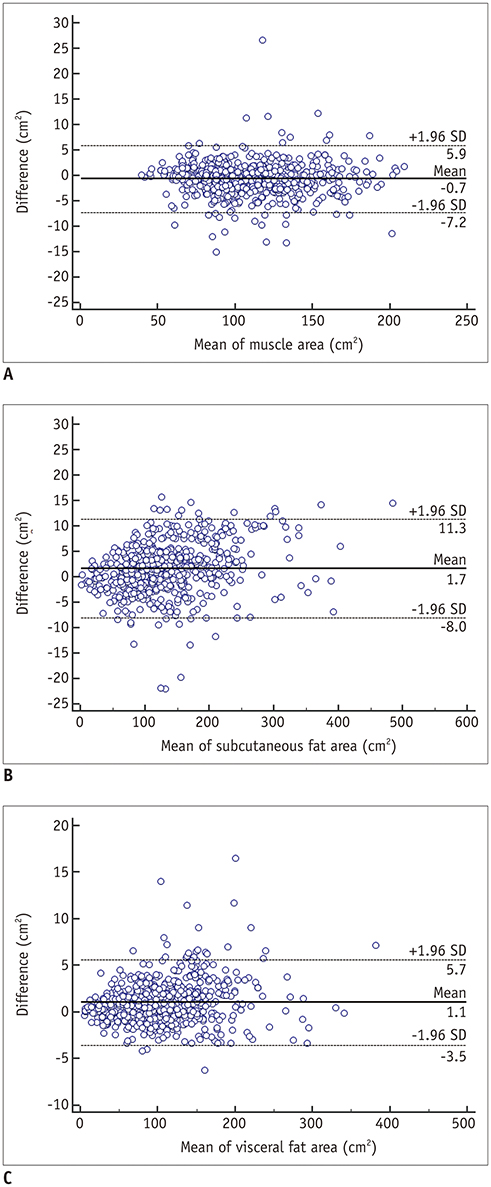
The mean Dice similarity coefficients for muscle, subcutaneous fat, and visceral fat were high for both the internal (0.96, 0.97, and 0.97, respectively) and external (0.97, 0.97, and 0.97, respectively) validation datasets, while the mean cross-sectional area errors for muscle, subcutaneous fat, and visceral fat were low for both internal (2.1%, 3.8%, and 1.8%, respectively) and external (2.7%, 4.6%, and 2.3%, respectively) validation datasets.
CONCLUSION
The fully convolutional network-based segmentation system exhibited high performance and accuracy in the automatic segmentation of abdominal muscle and fat on CT images.
MeSH Terms
Figure
Cited by 3 articles
-
Sex Differences of Visceral Fat Area and Visceral-to-Subcutaneous Fat Ratio for the Risk of Incident Type 2 Diabetes Mellitus
Eun Hee Kim, Hong-Kyu Kim, Min Jung Lee, Sung-Jin Bae, Jaewon Choe, Chang Hee Jung, Chul-Hee Kim, Joong-Yeol Park, Woo Je Lee
Diabetes Metab J. 2022;46(3):486-498. doi: 10.4093/dmj.2021.0095.Reference Values for Skeletal Muscle Mass at the Third Lumbar Vertebral Level Measured by Computed Tomography in a Healthy Korean Population
Ja Kyung Yoon, Sunyoung Lee, Kyoung Won Kim, Ji Eun Lee, Jeong Ah Hwang, Taeyong Park, Jeongjin Lee
Endocrinol Metab. 2021;36(3):672-677. doi: 10.3803/EnM.2021.1041.Differences in Abdominal Body Composition According to Glycemic Status: An Inverse Probability Treatment Weighting Analysis
Seungbong Han, Young-Jee Jeon, Gyung-Min Park, Tae Young Lee, Soon Eun Park, Gyeongseok Yu, Byung Ju Kang
Endocrinol Metab. 2021;36(4):855-864. doi: 10.3803/EnM.2021.1086.
Reference
-
1. Bosello O, Zamboni M. Visceral obesity and metabolic syndrome. Obes Rev. 2000; 1:47–56.
Article2. Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000; 21:697–738.
Article3. Blauwhoff-Buskermolen S, Versteeg KS, de van der Schueren MA, den Braver NR, Berkhof J, Langius JA, et al. Loss of muscle mass during chemotherapy is predictive for poor survival of patients with metastatic colorectal cancer. J Clin Oncol. 2016; 34:1339–1344.4. Kuroki LM, Mangano M, Allsworth JE, Menias CO, Massad LS, Powell MA, et al. Pre-operative assessment of muscle mass to predict surgical complications and prognosis in patients with endometrial cancer. Ann Surg Oncol. 2015; 22:972–979.
Article5. Reisinger KW, van Vugt JL, Tegels JJ, Snijders C, Hulsewé KW, Hoofwijk AG, et al. Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Ann Surg. 2015; 261:345–352.
Article6. Bokshan SL, Han AL, DePasse JM, Eltorai AE, Marcaccio SE, Palumbo MA, et al. Effect of sarcopenia on postoperative morbidity and mortality after thoracolumbar spine surgery. Orthopedics. 2016; 39:e1159–e1164.
Article7. Jones K, Gordon-Weeks A, Coleman C, Silva M. Radiologically determined sarcopenia predicts morbidity and mortality following abdominal surgery: a systematic review and meta-analysis. World J Surg. 2017; 41:2266–2279.
Article8. Fukuda Y, Yamamoto K, Hirao M, Nishikawa K, Nagatsuma Y, Nakayama T, et al. Sarcopenia is associated with severe postoperative complications in elderly gastric cancer patients undergoing gastrectomy. Gastric Cancer. 2016; 19:986–993.
Article9. Boutin RD, Yao L, Canter RJ, Lenchik L. Sarcopenia: current concepts and imaging implications. AJR Am J Roentgenol. 2015; 205:W255–W266.
Article10. Jones KI, Doleman B, Scott S, Lund JN, Williams JP. Simple psoas cross-sectional area measurement is a quick and easy method to assess sarcopenia and predicts major surgical complications. Colorectal Dis. 2015; 17:O20–O26.
Article11. Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008; 33:997–1006.
Article12. Shuster A, Patlas M, Pinthus JH, Mourtzakis M. The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. Br J Radiol. 2012; 85:1–10.
Article13. McDonald AM, Swain TA, Mayhew DL, Cardan RA, Baker CB, Harris DM, et al. CT measures of bone mineral density and muscle mass can be used to predict noncancer death in men with prostate cancer. Radiology. 2017; 282:475–483.
Article14. Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol (1985). 2004; 97:2333–2338.
Article15. Kamiya N, Zhou X, Chen H, Muramatsu C, Hara T, Yokoyama R, et al. Automated segmentation of psoas major muscle in X-ray CT images by use of a shape model: preliminary study. Radiol Phys Technol. 2012; 5:5–14.
Article16. Polan DF, Brady SL, Kaufman RA. Tissue segmentation of computed tomography images using a Random Forest algorithm: a feasibility study. Phys Med Biol. 2016; 61:6553–6569.
Article17. Chartrand G, Cheng PM, Vorontsov E, Drozdzal M, Turcotte S, Pal CJ, et al. Deep learning: a primer for radiologists. Radiographics. 2017; 37:2113–2131.
Article18. Lee H, Troschel FM, Tajmir S, Fuchs G, Mario J, Fintelmann FJ, et al. Pixel-level deep segmentation: artificial intelligence quantifies muscle on computed tomography for body morphometric analysis. J Digit Imaging. 2017; 30:487–498.
Article19. Burns JE, Yao J, Chalhoub D, Chen JJ, Summers RM. A machine learning algorithm to estimate sarcopenia on abdominal CT. Acad Radiol. 2019; pii: S1076-6332(19)30165-5.
Article20. Decazes P, Tonnelet D, Vera P, Gardin I. Anthropometer3D: automatic multi-slice segmentation software for the measurement of anthropometric parameters from CT of PET/CT. J Digit Imaging. 2019; 32:241–250.
Article21. Weston AD, Korfiatis P, Kline TL, Philbrick KA, Kostandy P, Sakinis T, et al. Automated abdominal segmentation of CT scans for body composition analysis using deep learning. Radiology. 2019; 290:669–679.
Article22. Hu P, Huo Y, Kong D, Carr JJ, Abramson RG, Hartley KG, et al. Automated characterization of body composition and frailty with clinically acquired CT. Comput Methods Clin Appl Musculoskelet Imaging (2017). 2018; 10734:25–35.
Article23. Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008; 9:629–635.
Article24. Shelhamer E, Long J, Darrell T. Fully convolutional networks for semantic segmentation. IEEE Trans Pattern Anal Mach Intell. 2017; 39:640–651.
Article25. Weisstein EW. Affine transformation. Wolfram MathWorld Web site. Published August 5, 2018. Accessed August 14, 2019. http://mathworld.wolfram.com/AffineTransformation.html.26. Larue RT, Defraene G, De Ruysscher D, Lambin P, van Elmpt W. Quantitative radiomics studies for tissue characterization: a review of technology and methodological procedures. Br J Radiol. 2017; 90:20160665.
Article27. Perona P, Malik J. Scale-space and edge detection using anisotropic diffusion. IEEE Trans Pattern Anal Mach Intell. 1990; 12:629–639.
Article28. Cropp RJ, Seslija P, Tso D, Thakur Y. Scanner and kVp dependence of measured CT numbers in the ACR CT phantom. J Appl Clin Med Phys. 2013; 14:4417.
Article29. Vala HJ, Baxi A. A review on Otsu image segmentation algorithm. IJARCET. 2013; 2:387–389.30. Otsu N. A threshold selection method from gray-level histograms. IEEE Trans Syst Man Cybern Syst. 1979; 9:62–66.
Article31. Dice LR. Measures of the amount of ecologic association between species. Ecology. 1945; 26:297–302.
Article32. Callahan LA, Supinski GS. Sepsis-induced myopathy. Crit Care Med. 2009; 37:S354.
Article33. Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent JL. Sepsis and septic shock. Nat Rev Dis Primers. 2016; 2:16045.
Article34. Bridge CP, Rosenthal M, Wright B, Kotecha G, Fintelmann F, Troschel F, et al. Fully-automated analysis of body composition from CT in cancer patients using convolutional neural networks. In : Stoyanov D, Taylor Z, Sarikaya D, McLeod J, Ballester MAG, Codella NCF, editors. OR 2.0 context-aware operating theaters, computer assisted robotic endoscopy, clinical image-based procedures, and skin image analysis. Cham: Springer;2018. p. 204–213.35. Popuri K, Cobzas D, Esfandiari N, Baracos V, Jägersand M. Body composition assessment in axial CT images using FEM-based automatic segmentation of skeletal muscle. IEEE Trans Med Imaging. 2016; 35:512–520.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Deep learning assisted biomarker development in patients with chronic hepatitis B: Editorial on “Prognostic role of computed tomography analysis using deep learning algorithm in patients with chronic hepatitis B viral infection”
- Automated Segmentation of Left Ventricular Myocardium on Cardiac Computed Tomography Using Deep Learning
- Current status of deep learning applications in abdominal ultrasonography
- Deep Learning in Dental Radiographic Imaging
- Development of an Optimized Deep Learning Model for Medical Imaging