Yonsei Med J.
2020 Jan;61(1):4-14. 10.3349/ymj.2020.61.1.4.
Update on the Management of Nonsteroidal Anti-Inflammatory Drug Hypersensitivity
- Affiliations
-
- 1Division of Rheumatology, Department of Internal Medicine, Pamela Youde Nethersole Eastern Hospital, Hong Kong, China.
- 2Department of Allergy and Clinical Immunology, Ajou University School of Medicine, Ajou University Medical Center, Suwon, Korea. hspark@ajou.ac.kr
- KMID: 2466330
- DOI: http://doi.org/10.3349/ymj.2020.61.1.4
Abstract
- The clinical phenotypes of nonsteroidal anti-inflammatory drug (NSAID) hypersensitivity are heterogeneous with various presentations including time of symptom onset, organ involvements, and underlying pathophysiology. Having a correct diagnosis can be challenging. Understanding their respective mechanisms as well as developing a comprehensive classification and diagnostic algorithm are pivotal for appropriate management strategy. Treatment modalities are based on the subtypes and severity of hypersensitivity reactions. Insights into the phenotypes and endotypes of hypersensitivity reactions enable personalized management in patients with suboptimal control of disease. This review updated the recent evidence of pathophysiology, classification, diagnostic algorithm, and management of NSAID hypersensitivity reactions.
Keyword
MeSH Terms
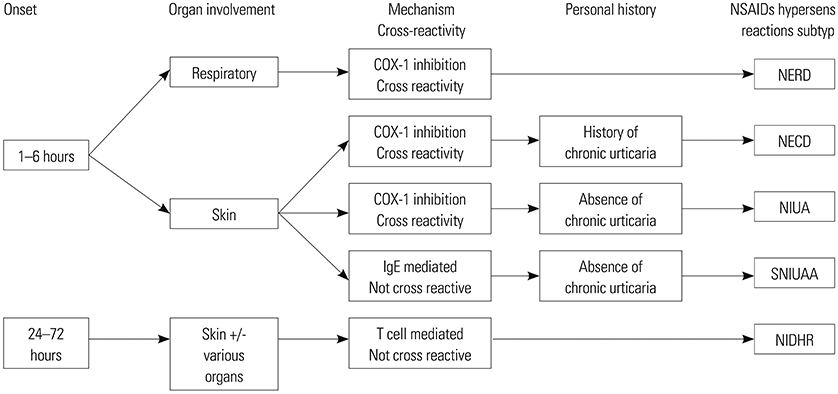
Figure
Reference
-
1. McGettigan P, Henry D. Use of non-steroidal anti-inflammatory drugs that elevate cardiovascular risk: an examination of sales and essential medicines lists in low-, middle-, and high-income countries. PLoS Med. 2013; 10:e1001388.
Article2. Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004; 329:15–19.
Article3. Iasella CJ, Johnson HJ, Dunn MA. Adverse drug reactions: type A (intrinsic) or type B (idiosyncratic). Clin Liver Dis. 2017; 21:73–87.4. Lafrance JP, Miller DR. Selective and non-selective non-steroidal anti-inflammatory drugs and the risk of acute kidney injury. Pharmacoepidemiol Drug Saf. 2009; 18:923–931.
Article5. Blumenthal KG, Lai KH, Huang M, Wallace ZS, Wickner PG, Zhou L. Adverse and hypersensitivity reactions to prescription nonsteroidal anti-Inflammatory agents in a large health care system. J Allergy Clin Immunol Pract. 2017; 5:737–743.
Article6. Thong BY. Nonsteroidal anti-inflammatory drug hypersensitivity in the Asia-Pacific. Asia Pac Allergy. 2018; 8:e38.
Article7. Lee JH, Jung CG, Park HS. An update on the management of aspirin-exacerbated respiratory disease. Expert Rev Respir Med. 2018; 12:137–143.
Article8. Kim YJ, Lim KH, Kim MY, Jo EJ, Lee SY, Lee SE, et al. Cross-reactivity to acetaminophen and celecoxib according to the type of nonsteroidal anti-inflammatory drug hypersensitivity. Allergy Asthma Immunol Res. 2014; 6:156–162.
Article9. Stone SF, Phillips EJ, Wiese MD, Heddle RJ, Brown SG. Immediate-type hypersensitivity drug reactions. Br J Clin Pharmacol. 2014; 78:1–13.
Article10. Kowalski ML, Makowska J. Reply: to PMID 24117484. Allergy. 2014; 69:815–816.11. Lee TH, Christie PE. Leukotrienes and aspirin induced asthma. Thorax. 1993; 48:1189–1190.
Article12. Divekar R, Hagan J, Rank M, Park M, Volcheck G, O’Brien E, et al. Diagnostic utility of urinary LTE4 in asthma, allergic rhinitis, chronic rhinosinusitis, nasal polyps, and aspirin sensitivity. J Allergy Clin Immunol Pract. 2016; 4:665–670.
Article13. Gaber F, Daham K, Higashi A, Higashi N, Gülich A, Delin I, et al. Increased levels of cysteinyl-leukotrienes in saliva, induced sputum, urine and blood from patients with aspirin-intolerant asthma. Thorax. 2008; 63:1076–1082.
Article14. Liu T, Laidlaw TM, Katz HR, Boyce JA. Prostaglandin E2 deficiency causes a phenotype of aspirin sensitivity that depends on platelets and cysteinyl leukotrienes. Proc Natl Acad Sci U S A. 2013; 110:16987–16992.
Article15. Steinke JW, Negri J, Liu L, Payne SC, Borish L. Aspirin activation of eosinophils and mast cells: implications in the pathogenesis of aspirin-exacerbated respiratory disease. J Immunol. 2014; 193:41–47.
Article16. Buchheit KM, Cahill KN, Katz HR, Murphy KC, Feng C, Lee-Sarwar K, et al. Thymic stromal lymphopoietin controls prostaglandin D2 generation in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2016; 137:1566–1576.
Article17. Choi Y, Lee Y, Park HS. Which factors associated with activated eosinophils contribute to the pathogenesis of aspirin-exacerbated respiratory disease? Allergy Asthma Immunol Res. 2019; 11:320–329.
Article18. Lee JU, Park JS, Chang HS, Park CS. Complementary participation of genetics and epigenetics in development of NSAID-exacerbated respiratory disease. Allergy Asthma Immunol Res. 2019; 11:779–794.
Article19. Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018; 73:1393–1414.20. Kowalski ML, Stevenson DD. Classification of reactions to nonsteroidal antiinflammatory drugs. Immunol Allergy Clin North Am. 2013; 33:135–145.
Article21. Szczeklik A, Sanak M. The broken balance in aspirin hypersensitivity. Eur J Pharmacol. 2006; 533:145–155.
Article22. Doña I, Jurado-Escobar R, Perkins JR, Ayuso P, Plaza-Serón MC, Pérez-Sánchez N, et al. Eicosanoid mediator profiles in different phenotypes of nonsteroidal anti-inflammatory drug-induced urticaria. Allergy. 2019; 74:1135–1144.
Article23. Hsieh CW, Lee JW, Liao EC, Tsai JJ. A disease marker for aspirin-induced chronic urticaria. Int J Mol Sci. 2014; 15:12591–12603.
Article24. Bae JS, Kim SH, Ye YM, Yoon HJ, Suh CH, Nahm DH, et al. Significant association of FcepsilonRIalpha promoter polymorphisms with aspirin-intolerant chronic urticaria. J Allergy Clin Immunol. 2007; 119:449–456.
Article25. Kim SH, Sanak M, Park HS. Genetics of hypersensitivity to aspirin and nonsteroidal anti-inflammatory drugs. Immunol Allergy Clin North Am. 2013; 33:177–194.
Article26. van der Klauw MM, Wilson JH, Stricker BH. Drug-associated anaphylaxis: 20 years of reporting in The Netherlands (1974–1994) and review of the literature. Clin Exp Allergy. 1996; 26:1355–1363.
Article27. Posadas SJ, Pichler WJ. Delayed drug hypersensitivity reactions -new concepts. Clin Exp Allergy. 2007; 37:989–999.
Article28. Mockenhaupt M, Kelly JP, Kaufman D, Stern RS. SCAR Study Group. The risk of Stevens-Johnson syndrome and toxic epidermal necrolysis associated with nonsteroidal antiinflammatory drugs: a multinational perspective. J Rheumatol. 2003; 30:2234–2240.29. Lee SY, Nam YH, Koh YI, Kim SH, Kim S, Kang HR, et al. Phenotypes of severe cutaneous adverse reactions caused by nonsteroidal anti-inflammatory drugs. Allergy Asthma Immunol Res. 2019; 11:212–221.
Article30. Malskat WS, Knulst AC, Bruijnzeel-Koomen CA, Röckmann H. Tolerance to alternative cyclooxygenase-2 inhibitors in nonsteroidal anti-inflammatory drug hypersensitive patients. Clin Transl Allergy. 2013; 3:20.
Article31. Liccardi G, Salzillo A, Piccolo A, Senna G, Piscitelli E, D’Amato M, et al. Safety of celecoxib in patients with adverse skin reactions to acetaminophen (paracetamol) and nimesulide associated or not with common non-steroidal anti-inflammatory drugs. Eur Ann Allergy Clin Immunol. 2005; 37:50–53.32. Sánchez-Borges M, Capriles-Hulett A. Atopy is a risk factor for non-steroidal anti-inflammatory drug sensitivity. Ann Allergy Asthma Immunol. 2000; 84:101–106.
Article33. Asero R. Single NSAID hypersensitivity is associated with atopic status. Eur Ann Allergy Clin Immunol. 2015; 47:48–53.34. Demoly P, Adkinson NF, Brockow K, Castells M, Chiriac AM, Greenberger PA, et al. International consensus on drug allergy. Allergy. 2014; 69:420–437.
Article35. Sánchez-Borges M, Caballero-Fonseca F, Capriles-Hulett A. Cofactors and comorbidities in patients with aspirin/NSAID hypersensitivity. Allergol Immunopathol (Madr). 2017; 45:573–578.
Article36. Lee HY, Ye YM, Kim SH, Ban GY, Kim SC, Kim JH, et al. Identification of phenotypic clusters of nonsteroidal anti-inflammatory drugs exacerbated respiratory disease. Allergy. 2017; 72:616–626.
Article37. Blanca-Lopez N, J Torres M, Doña I, Campo P, Rondón C, Seoane Reula ME, et al. Value of the clinical history in the diagnosis of urticaria/angioedema induced by NSAIDs with cross-intolerance. Clin Exp Allergy. 2013; 43:85–91.
Article38. Sánchez-Borges M, Fernández-Caldas E, Capriles-Hulett A, Caballero-Fonseca F. Mite-induced inflammation: more than allergy. Allergy Rhinol (Providence). 2012; 3:e25–e29.
Article39. Kowalski ML, Makowska JS. Seven steps to the diagnosis of NSAIDs hypersensitivity: how to apply a new classification in real practice? Allergy Asthma Immunol Res. 2015; 7:312–320.
Article40. Hoetzenecker W, Nägeli M, Mehra ET, Jensen AN, Saulite I, Schmid-Grendelmeier P, et al. Adverse cutaneous drug eruptions: current understanding. Semin Immunopathol. 2016; 38:75–86.
Article41. Himly M, Jahn-Schmid B, Pittertschatscher K, Bohle B, Grubmayr K, Ferreira F, et al. IgE-mediated immediate-type hypersensitivity to the pyrazolone drug propyphenazone. J Allergy Clin Immunol. 2003; 111:882–888.
Article42. Brockow K, Garvey LH, Aberer W, Atanaskovic-Markovic M, Barbaud A, Bilo MB, et al. Skin test concentrations for systemically administered drugs -- an ENDA/EAACI Drug Allergy Interest Group position paper. Allergy. 2013; 68:702–712.
Article43. Kang MG, Sohn KH, Kang DY, Park HK, Yang MS, Lee JY, et al. Analysis of individual case safety reports of severe cutaneous adverse reactions in Korea. Yonsei Med J. 2019; 60:208–215.
Article44. Kowalski ML, Makowska JS, Blanca M, Bavbek S, Bochenek G, Bousquet J, et al. Hypersensitivity to nonsteroidal anti-inflammatory drugs (NSAIDs) - classification, diagnosis and management: review of the EAACI/ENDA and GA2LEN/HANNA. Allergy. 2011; 66:818–829.
Article45. DeGregorio GA, Singer J, Cahill KN, Laidlaw T. A 1-Day, 90-minute aspirin challenge and desensitization protocol in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol Pract. 2019; 7:1174–1180.
Article46. Kowalski ML, Agache I, Bavbek S, Bakirtas A, Blanca M, Bochenek G, et al. Diagnosis and management of NSAID-Exacerbated Respiratory Disease (N-ERD)-a EAACI position paper. Allergy. 2019; 74:28–39.
Article47. Kowalski ML, Woessner K, Sanak M. Approaches to the diagnosis and management of patients with a history of nonsteroidal anti-inflammatory drug-related urticaria and angioedema. J Allergy Clin Immunol. 2015; 136:245–251.
Article48. Zisa G, Riccobono F, Bommarito L, D’Antonio C, Calamari AM, Poppa M, et al. Provocation tests with the offending nonsteroidal anti-inflammatory drugs in patients with urticaria/angioedema reactions. Allergy Asthma Proc. 2012; 33:421–426.
Article49. Kowalski ML, Asero R, Bavbek S, Blanca M, Blanca-Lopez N, Bochenek G, et al. Classification and practical approach to the diagnosis and management of hypersensitivity to nonsteroidal anti-inflammatory drugs. Allergy. 2013; 68:1219–1232.
Article50. Aberer W, Bircher A, Romano A, Blanca M, Campi P, Fernandez J, et al. Drug provocation testing in the diagnosis of drug hypersensitivity reactions: general considerations. Allergy. 2003; 58:854–863.
Article51. Sanz ML, Sánchez G, Gamboa PM, Vila L, Uasuf C, Chazot M, et al. Allergen-induced basophil activation: CD63 cell expression detected by flow cytometry in patients allergic to Dermatophagoides pteronyssinus and Lolium perenne. Clin Exp Allergy. 2001; 31:1007–1013.
Article52. Gamboa P, Sanz ML, Caballero MR, Urrutia I, Antépara I, Esparza R, et al. The flow-cytometric determination of basophil activation induced by aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs) is useful for in vitro diagnosis of the NSAID hypersensitivity syndrome. Clin Exp Allergy. 2004; 34:1448–1457.
Article53. Hemmings O, Kwok M, McKendry R, Santos AF. Basophil activation test: old and new applications in allergy. Curr Allergy Asthma Rep. 2018; 18:77.
Article54. Hagan JB, Laidlaw TM, Divekar R, O’Brien EK, Kita H, Volcheck GW, et al. Urinary leukotriene E4 to determine aspirin intolerance in asthma: a systematic review and meta-analysis. J Allergy Clin Immunol Pract. 2017; 5:990–997.
Article55. Choi Y, Lee DH, Trinh HKT, Ban GY, Park HK, Shin YS, et al. Surfactant protein D alleviates eosinophil-mediated airway inflammation and remodeling in patients with aspirin-exacerbated respiratory disease. Allergy. 2019; 74:78–88.
Article56. Trinh HKT, Pham DL, Choi Y, Kim HM, Kim SH, Park HS. Epithelial folliculin enhances airway inflammation in aspirin-exacerbated respiratory disease. Clin Exp Allergy. 2018; 48:1464–1473.
Article57. Wardzyńska A, Makowska JS, Pawełczyk M, Piechota-Polańczyk A, Kurowski M, Kowalski ML. Periostin in exhaled breath condensate and in serum of asthmatic patients: relationship to upper and lower airway disease. Allergy Asthma Immunol Res. 2017; 9:126–132.
Article58. Pichler WJ, Tilch J. The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy. 2004; 59:809–820.
Article59. Agache I, Rogozea L. Asthma biomarkers: do they bring precision medicine closer to the clinic? Allergy Asthma Immunol Res. 2017; 9:466–476.
Article60. Wöhrl S. NSAID hypersensitivity - recommendations for diagnostic work up and patient management. Allergo J Int. 2018; 27:114–121.
Article61. Reddel HK, FitzGerald JM, Bateman ED, Bacharier LB, Becker A, Brusselle G, et al. GINA 2019: a fundamental change in asthma management: treatment of asthma with short-acting bronchodilators alone is no longer recommended for adults and adolescents. Eur Respir J. 2019; 53:1901046.62. Marcello C, Carlo L. Asthma phenotypes: the intriguing selective intervention with Montelukast. Asthma Res Pract. 2016; 2:11.
Article63. Ta V, White AA. Survey-defined patient experiences with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol Pract. 2015; 3:711–718.
Article64. Mastalerz L, Nizankowska E, Sanak M, Mejza F, Pierzchalska M, Bazan-Socha S, et al. Clinical and genetic features underlying the response of patients with bronchial asthma to treatment with a leukotriene receptor antagonist. Eur J Clin Invest. 2002; 32:949–955.
Article65. Hanania NA, Alpan O, Hamilos DL, Condemi JJ, Reyes-Rivera I, Zhu J, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med. 2011; 154:573–582.
Article66. Bardelas J, Figliomeni M, Kianifard F, Meng X. A 26-week, randomized, double-blind, placebo-controlled, multicenter study to evaluate the effect of omalizumab on asthma control in patients with persistent allergic asthma. J Asthma. 2012; 49:144–152.
Article67. Hayashi H, Mitsui C, Nakatani E, Fukutomi Y, Kajiwara K, Watai K, et al. Omalizumab reduces cysteinyl leukotriene and 9α,11β-prostaglandin F2 overproduction in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2016; 137:1585–1587.68. Jean T, Eng V, Sheikh J, Kaplan MS, Goldberg B, Jau Yang S, et al. Effect of omalizumab on outcomes in patients with aspirin-exacerbated respiratory disease. Allergy Asthma Proc. 2019; 40:316–320.
Article69. Lee JH, Lee HY, Jung CG, Ban GY, Shin YS, Ye YM, et al. Therapeutic effect of omalizumab in severe asthma: a real-world study in Korea. Allergy Asthma Immunol Res. 2018; 10:121–130.
Article70. Porcaro F, Di Marco A, Cutrera R. Omalizumab in patient with aspirin exacerbated respiratory disease and chronic idiopathic urticaria. Pediatr Pulmonol. 2017; 52:E26–E28.
Article71. Guillén D, Bobolea I, Calderon O, Fiandor A, Cabañas R, Heredia R, et al. Aspirin desensitization achieved after omalizumab treatment in a patient with aspirin-exacerbated urticaria and respiratory disease. J Investig Allergol Clin Immunol. 2015; 25:133–135.72. Yorgancıoğlu A, Öner Erkekol F, Mungan D, Erdinç M, Gemicioğlu B, Özs¸eker ZF, et al. Long-term omalizumab treatment: a multicenter, real-life, 5-year trial. Int Arch Allergy Immunol. 2018; 176:225–233.
Article73. Varricchi G, Bagnasco D, Borriello F, Heffler E, Canonica GW. Interleukin-5 pathway inhibition in the treatment of eosinophilic respiratory disorders: evidence and unmet needs. Curr Opin Allergy Clin Immunol. 2016; 16:186–200.
Article74. Emma R, Morjaria JB, Fuochi V, Polosa R, Caruso M. Mepolizumab in the management of severe eosinophilic asthma in adults: current evidence and practical experience. Ther Adv Respir Dis. 2018; 12:1753466618808490.
Article75. Tuttle KL, Buchheit KM, Laidlaw TM, Cahill KN. A retrospective analysis of mepolizumab in subjects with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol Pract. 2018; 6:1045–1047.
Article76. Liddament M, Husten J, Estephan T, Laine D, Mabon D, Pukac L, et al. Higher binding affinity and in vitro potency of reslizumab for interleukin-5 compared with mepolizumab. Allergy Asthma Immunol Res. 2019; 11:291–298.
Article77. Laidlaw TM, Mullol J, Fan C, Zhang D, Amin N, Khan A, et al. Dupilumab improves nasal polyp burden and asthma control in patients with CRSwNP and AERD. J Allergy Clin Immunol Pract. 2019; 7:2462–2465.
Article78. Gauvreau GM, O'Byrne PM, Boulet LP, Wang Y, Cockcroft D, Bigler J, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med. 2014; 370:2102–2110.
Article79. Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012; 50:1–12.
Article80. Howard BE, Lal D. Oral steroid therapy in chronic rhinosinusitis with and without nasal polyposis. Curr Allergy Asthma Rep. 2013; 13:236–243.
Article81. Hamilos DL. Chronic rhinosinusitis: epidemiology and medical management. J Allergy Clin Immunol. 2011; 128:693–707.
Article82. Mendelsohn D, Jeremic G, Wright ED, Rotenberg BW. Revision rates after endoscopic sinus surgery: a recurrence analysis. Ann Otol Rhinol Laryngol. 2011; 120:162–166.
Article83. Stryjewska-Makuch G, Humeniuk-Arasiewicz M, Jura-Szołtys E, Glück J. The effect of antileukotrienes on the results of postoperative treatment of paranasal sinuses in patients with non-steroidal anti-inflammatory drug-exacerbated respiratory disease. Int Arch Allergy Immunol. 2019; 179:281–289.
Article84. Kim DK, Kim DW. Does inflammatory endotype change in patients with chronic rhinosinusitis? Allergy Asthma Immunol Res. 2019; 11:153–155.
Article85. Naidoo Y, Bassiouni A, Keen M, Wormald PJ. Long-term outcomes for the endoscopic modified Lothrop/Draf III procedure: a 10-year review. Laryngoscope. 2014; 124:43–49.
Article86. Fandiño M, Macdonald KI, Lee J, Witterick IJ. The use of postoperative topical corticosteroids in chronic rhinosinusitis with nasal polyps: a systematic review and meta-analysis. Am J Rhinol Allergy. 2013; 27:e146–e157.87. Van Gerven L, Langdon C, Cordero A, Cardelús S, Mullol J, Alobid I. Lack of long-term add-on effect by montelukast in postoperative chronic rhinosinusitis patients with nasal polyps. Laryngoscope. 2018; 128:1743–1751.
Article88. Chandra RK, Clavenna M, Samuelson M, Tanner SB, Turner JH. Impact of omalizumab therapy on medication requirements for chronic rhinosinusitis. Int Forum Allergy Rhinol. 2016; 6:472–477.
Article89. Gevaert P, Calus L, Van Zele T, Blomme K, De Ruyck N, Bauters W, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol. 2013; 131:110–116.
Article90. Rivero A, Liang J. Anti-IgE and anti-IL5 biologic therapy in the treatment of nasal polyposis: a systematic review and meta-analysis. Ann Otol Rhinol Laryngol. 2017; 126:739–747.
Article91. Bachert C, Mannent L, Naclerio RM, Mullol J, Ferguson BJ, Gevaert P, et al. Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a randomized clinical trial. JAMA. 2016; 315:469–479.
Article92. Hill J, Burnett T, Katial R. Mechanisms of benefit with aspirin therapy in aspirin-exacerbated respiratory disease. Immunol Allergy Clin North Am. 2016; 36:735–747.
Article93. Perez-G M, Melo M, Keegan AD, Zamorano J. Aspirin and salicylates inhibit the IL-4- and IL-13-induced activation of STAT6. J Immunol. 2002; 168:1428–1434.
Article94. Kong SK, Kim BS, Uhm TG, Chang HS, Park JS, Park SW, et al. Aspirin induces IL-4 production: augmented IL-4 production in aspirin-exacerbated respiratory disease. Exp Mol Med. 2016; 48:e202.
Article95. White AA, Stevenson DD. Aspirin desensitization: faster protocols for busy patients. J Allergy Clin Immunol Pract. 2019; 7:1181–1183.
Article96. Berges-Gimeno MP, Simon RA, Stevenson DD. Long-term treatment with aspirin desensitization in asthmatic patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2003; 111:180–186.
Article97. Walters KM, Waldram JD, Woessner KM, White AA. Long-term clinical outcomes of aspirin desensitization with continuous daily aspirin therapy in aspirin-exacerbated respiratory disease. Am J Rhinol Allergy. 2018; 32:280–286.
Article98. Maurer M, Sofen H, Ortiz B, Kianifard F, Gabriel S, Bernstein JA. Positive impact of omalizumab on angioedema and quality of life in patients with refractory chronic idiopathic/spontaneous urticaria: analyses according to the presence or absence of angioedema. J Eur Acad Dermatol Venereol. 2017; 31:1056–1063.
Article99. Ring J, Beyer K, Biedermann T, Bircher A, Duda D, Fischer J, et al. Guideline for acute therapy and management of anaphylaxis: S2 Guideline of the German Society for Allergology and Clinical Immunology (DGAKI), the Association of German Allergologists (AeDA), the Society of Pediatric Allergy and Environmental Medicine (GPA), the German Academy of Allergology and Environmental Medicine (DAAU), the German Professional Association of Pediatricians (BVKJ), the Austrian Society for Allergology and Immunology (ÖGAI), the Swiss Society for Allergy and Immunology (SGAI), the German Society of Anaesthesiology and Intensive Care Medicine (DGAI), the German Society of Pharmacology (DGP), the German Society for Psychosomatic Medicine (DGPM), the German Working Group of Anaphylaxis Training and Education (AGATE) and the patient organization German Allergy and Asthma Association (DAAB). Allergo J Int. 2014; 23:96–112.
Article100. Scherer K, Brockow K, Aberer W, Gooi JH, Demoly P, Romano A, et al. Desensitization in delayed drug hypersensitivity reactions -- an EAACI position paper of the Drug Allergy Interest Group. Allergy. 2013; 68:844–852.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hypersensitivity to Aspirin and Nonsteroidal Anti-inflammatory Drugs
- Seven Steps to the Diagnosis of NSAIDs Hypersensitivity: How to Apply a New Classification in Real Practice?
- Tolerance to etoricoxib in children with nonsteroidal anti-inflammatory drug hypersensitivity
- A single nonsteroidal anti-inflammatory drugs-induced anaphylaxis to diclofenac confirmed by skin testing
- Two cases of hypersensitivity to isopropylantipyrine