J Gastric Cancer.
2019 Dec;19(4):438-450. 10.5230/jgc.2019.19.e42.
Feasibility of Linear-Shaped Gastroduodenostomy during the Performance of Totally Robotic Distal Gastrectomy
- Affiliations
-
- 1Department of Surgery, Ajou University School of Medicine, Suwon, Korea. hansu@ajou.ac.kr
- KMID: 2466032
- DOI: http://doi.org/10.5230/jgc.2019.19.e42
Abstract
- PURPOSE
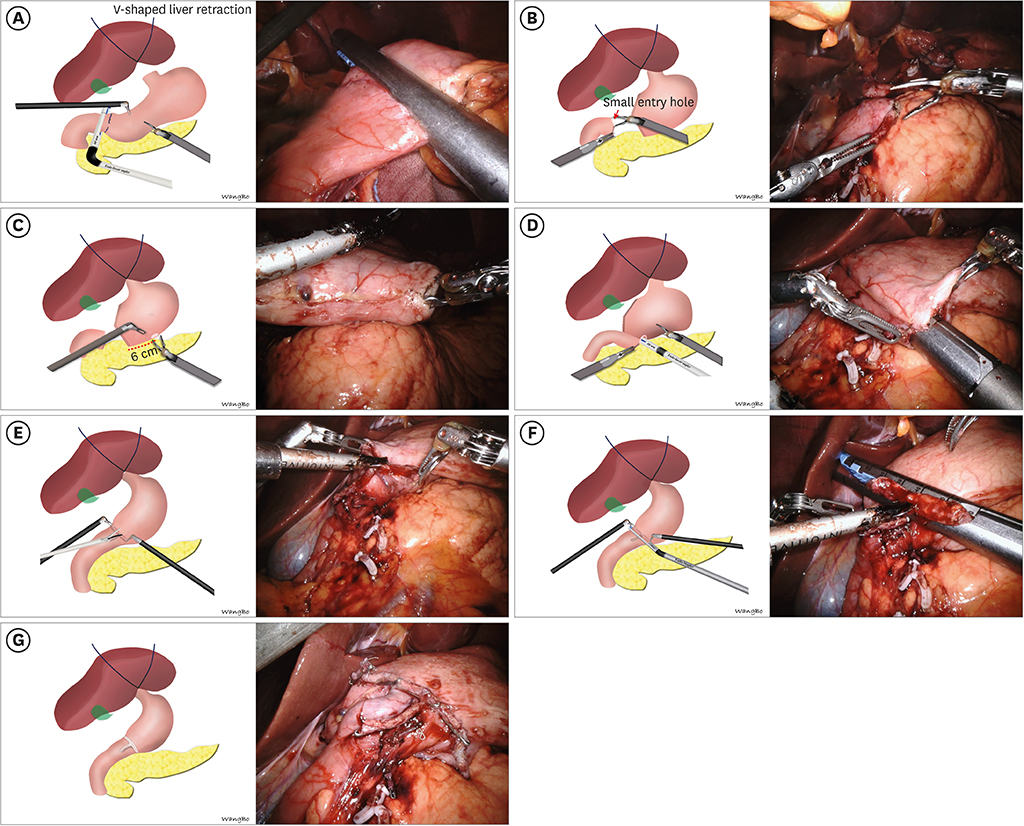
Although linear-shaped gastroduodenostomy (LSGD) was reported to be a feasible and reliable method of Billroth I anastomosis in patients undergoing totally laparoscopic distal gastrectomy (TLDG), the feasibility of LSGD for patients undergoing totally robotic distal gastrectomy (TRDG) has not been determined. This study compared the feasibility of LSGD in patients undergoing TRDG and TLDG.
MATERIALS AND METHODS
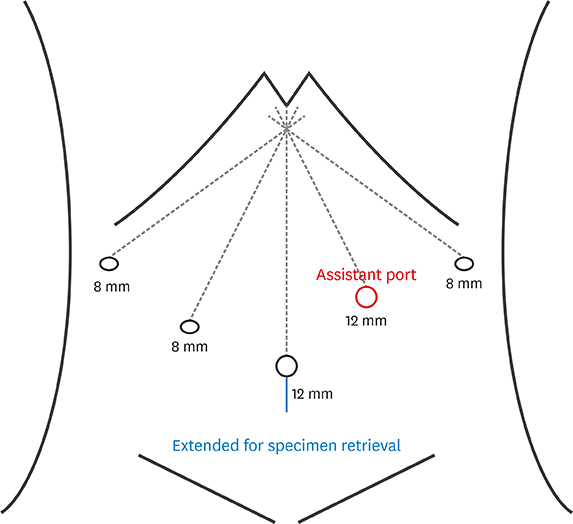
ALL C: onsecutive patients who underwent LSGD after distal gastrectomy for gastric cancer between January 2009 and December 2017 were analyzed retrospectively. Propensity score matching (PSM) analysis was performed to reduce the selection bias between TRDG and TLDG. Short-term outcomes, functional outcomes, learning curve, and risk factors for postoperative complications were analyzed.
RESULTS
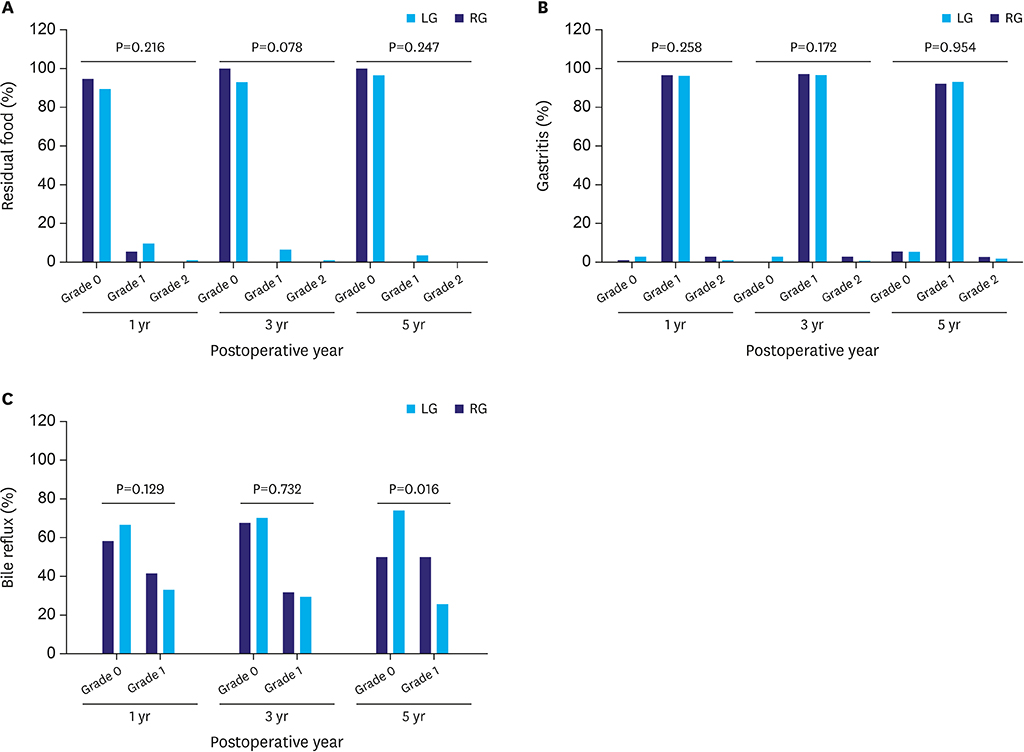
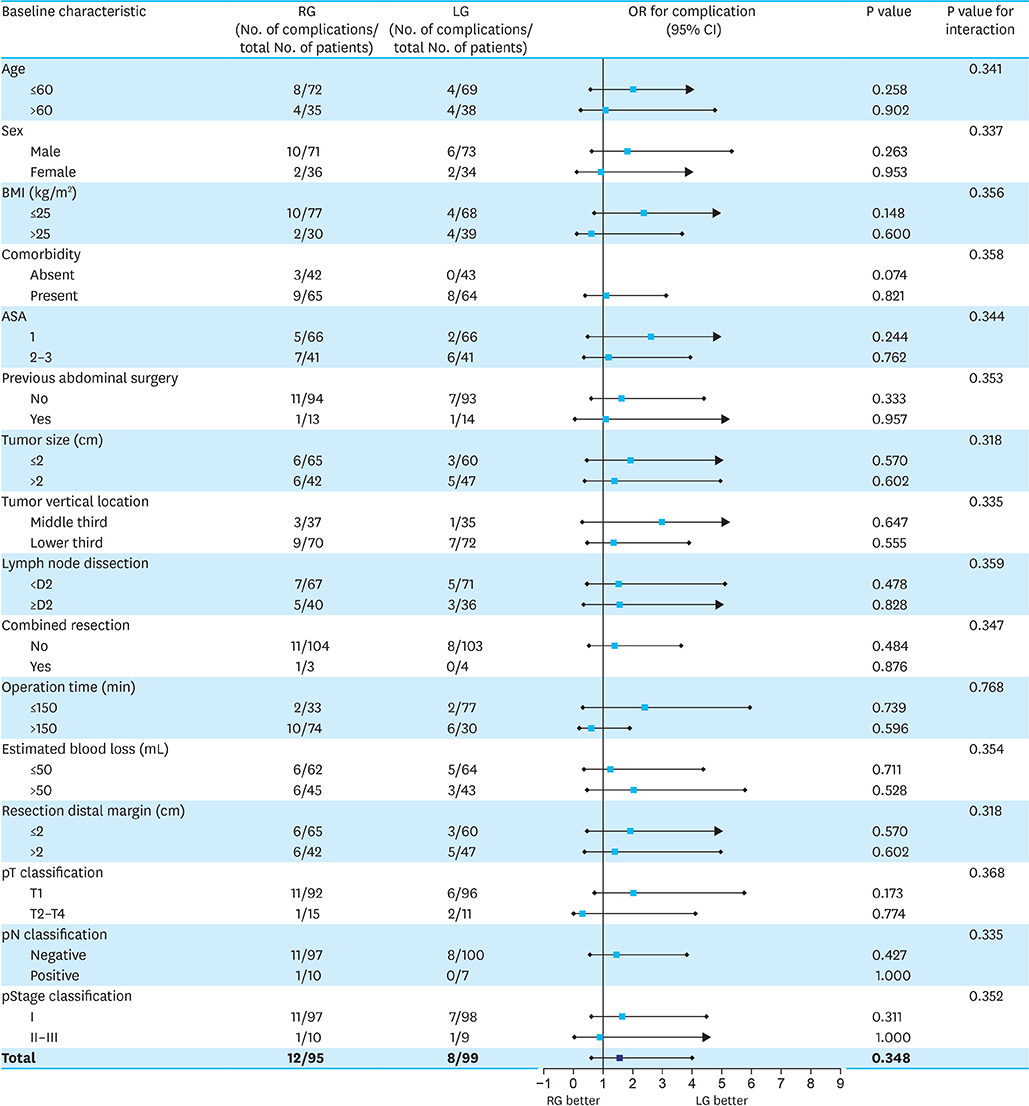
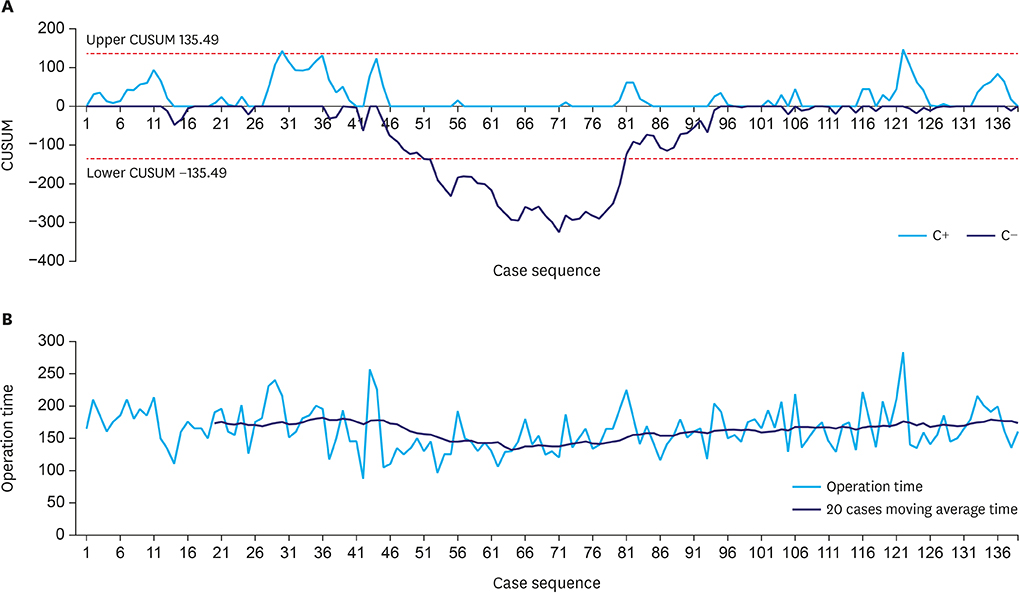
This analysis included 414 patients, of whom 275 underwent laparoscopy and 139 underwent robotic surgery. PSM analysis showed that operation time was significantly longer (163.5 vs. 132.1 minutes, P<0.001) and postoperative hospital stay significantly shorter (6.2 vs. 7.5 days, P<0.003) in patients who underwent TRDG than in patients who underwent TLDG. Operation time was the independent risk factor for LSGD after intracorporeal gastroduodenostomy. Cumulative sum analysis showed no definitive turning point in the TRDG learning curve. Long-term endoscopic findings revealed similar results in the two groups, but bile reflux at 5 years showed significantly better improvement in the TLDG group than in the TRDG group (P=0.016).
CONCLUSIONS
LSGD is feasible in TRDG, with short-term and long-term outcomes comparable to that in TLDG. LSGD may be a good option for intracorporeal Billroth I anastomosis in patients undergoing TRDG.
MeSH Terms
Figure
Reference
-
1. Hashizume M, Shimada M, Tomikawa M, Ikeda Y, Takahashi I, Abe R, et al. Early experiences of endoscopic procedures in general surgery assisted by a computer-enhanced surgical system. Surg Endosc. 2002; 16:1187–1191.2. Alhossaini RM, Altamran AA, Seo WJ, Hyung WJ. Robotic gastrectomy for gastric cancer: current evidence. Ann Gastroenterol Surg. 2017; 1:82–89.3. Liu XX, Jiang ZW, Chen P, Zhao Y, Pan HF, Li JS. Full robot-assisted gastrectomy with intracorporeal robot-sewn anastomosis produces satisfying outcomes. World J Gastroenterol. 2013; 19:6427–6437.4. Parisi A, Ricci F, Trastulli S, Cirocchi R, Gemini A, Grassi V, et al. Robotic total gastrectomy with intracorporeal robot-sewn anastomosis: a novel approach adopting the double-loop reconstruction method. Medicine (Baltimore). 2015; 94:e1922.5. Ikeda O, Sakaguchi Y, Aoki Y, Harimoto N, Taomoto J, Masuda T, et al. Advantages of totally laparoscopic distal gastrectomy over laparoscopically assisted distal gastrectomy for gastric cancer. Surg Endosc. 2009; 23:2374–2379.6. Lee SW, Tanigawa N, Nomura E, Tokuhara T, Kawai M, Yokoyama K, et al. Benefits of intracorporeal gastrointestinal anastomosis following laparoscopic distal gastrectomy. World J Surg Oncol. 2012; 10:267.7. Hirao M, Takiguchi S, Imamura H, Yamamoto K, Kurokawa Y, Fujita J, et al. Comparison of Billroth I and Roux-en-Y reconstruction after distal gastrectomy for gastric cancer: one-year postoperative effects assessed by a multi-institutional RCT. Ann Surg Oncol. 2013; 20:1591–1597.8. Kang KM, Cho YS, Min SH, Lee Y, Park KB, Park YS, et al. Internal hernia after gastrectomy for gastric cancer in minimally invasive surgery era. Gastric Cancer. 2019; 22:1009–1015.9. Kanaya S, Gomi T, Momoi H, Tamaki N, Isobe H, Katayama T, et al. Delta-shaped anastomosis in totally laparoscopic Billroth I gastrectomy: new technique of intraabdominal gastroduodenostomy. J Am Coll Surg. 2002; 195:284–287.10. Okabe H, Obama K, Tsunoda S, Tanaka E, Sakai Y. Advantage of completely laparoscopic gastrectomy with linear stapled reconstruction: a long-term follow-up study. Ann Surg. 2014; 259:109–116.11. Noshiro H, Iwasaki H, Miyasaka Y, Kobayashi K, Masatsugu T, Akashi M, et al. An additional suture secures against pitfalls in delta-shaped gastroduodenostomy after laparoscopic distal gastrectomy. Gastric Cancer. 2011; 14:385–389.12. Kitagami H, Nonoyama K, Yasuda A, Kurashima Y, Watanabe K, Fujihata S, et al. Technique of totally robotic delta-shaped anastomosis in distal gastrectomy. J Minim Access Surg. 2017; 13:215–218.13. Kikuchi K, Suda K, Nakauchi M, Shibasaki S, Nakamura K, Kajiwara S, et al. Delta-shaped anastomosis in totally robotic Billroth I gastrectomy: technical aspects and short-term outcomes. Asian J Endosc Surg. 2016; 9:250–257.14. Lee JH, Son T, Kim J, Seo WJ, Rho CK, Cho M, et al. Intracorporeal delta-shaped gastroduodenostomy in reduced-port robotic distal subtotal gastrectomy: technical aspects and short-term outcomes. Surg Endosc. 2018; 32:4344–4350.15. Song HM, Lee SL, Hur H, Cho YK, Han SU. Linear-shaped gastroduodenostomy in totally laparoscopic distal gastrectomy. J Gastric Cancer. 2010; 10:69.16. Wang B, Son SY, Shin HJ, Hur H, Han SU. The learning curve of linear-shaped gastroduodenostomy associated with totally laparoscopic distal gastrectomy. J Gastrointest Surg. 2019.17. Byun C, Cui LH, Son SY, Hur H, Cho YK, Han SU. Linear-shaped gastroduodenostomy (LSGD): safe and feasible technique of intracorporeal Billroth I anastomosis. Surg Endosc. 2016; 30:4505–4514.18. Oh DK, Hur H, Kim JY, Han SU, Cho YK. V-shaped liver retraction during a laparoscopic gastrectomy for gastric cancer. J Gastric Cancer. 2010; 10:133.19. Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011; 14:101–112.20. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011; 14:113–123.21. Kubo M, Sasako M, Gotoda T, Ono H, Fujishiro M, Saito D, et al. Endoscopic evaluation of the remnant stomach after gastrectomy: proposal for a new classification. Gastric Cancer. 2002; 5:83–89.22. Wang Y, Zhao X, Song Y, Cai A, Xi H, Chen L. A systematic review and meta-analysis of robot-assisted versus laparoscopically assisted gastrectomy for gastric cancer. Medicine (Baltimore). 2017; 96:e8797.23. Cestari A, Ferrari M, Zanoni M, Sangalli M, Ghezzi M, Fabbri F, et al. Side docking of the da Vinci robotic system for radical prostatectomy: advantages over traditional docking. J Robot Surg. 2015; 9:243–247.24. Berber E, Mitchell J, Milas M, Siperstein A. Robotic posterior retroperitoneal adrenalectomy: operative technique. Arch Surg. 2010; 145:781–784.25. Cheng H, Clymer JW, Po-Han Chen B, Sadeghirad B, Ferko NC, Cameron CG, et al. Prolonged operative duration is associated with complications: a systematic review and meta-analysis. J Surg Res. 2018; 229:134–144.26. Phan K, Kim JS, Capua JD, Lee NJ, Kothari P, Dowdell J, et al. Impact of operation time on 30-day complications after adult spinal deformity surgery. Global Spine J. 2017; 7:664–671.27. An JY, Kim SM, Ahn S, Choi MG, Lee JH, Sohn TS, et al. Successful robotic gastrectomy does not require extensive laparoscopic experience. J Gastric Cancer. 2018; 18:90–98.28. Park SS, Kim MC, Park MS, Hyung WJ. Rapid adaptation of robotic gastrectomy for gastric cancer by experienced laparoscopic surgeons. Surg Endosc. 2012; 26:60–67.29. Hong SS, Son SY, Shin HJ, Cui LH, Hur H, Han SU. Can Robotic gastrectomy surpass laparoscopic gastrectomy by acquiring long-term experience? A propensity score analysis of a 7-year experience at a single institution. J Gastric Cancer. 2016; 16:240–246.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Linear-Shaped Gastroduodenostomy in Totally Laparoscopic Distal Gastrectomy
- Delta-Shaped Gastroduodenostomy after Totally Laparoscopic Distal Gastrectomy: A Comparison Analysis between Early and Late Experience
- The Early Experience with a Totally Laparoscopic Distal Gastrectomy
- Intracorporeal Anastomosis in Laparoscopic Gastric Cancer Surgery
- Comparison of Laparoscopy-Assisted and Totally Laparoscopic Distal Gastrectomy: The Short-Term Outcome at a Low Volume Center