Clin Endosc.
2019 Nov;52(6):588-597. 10.5946/ce.2019.018.
Predictive Value of Localized Stenosis of the Main Pancreatic Duct for Early Detection of Pancreatic Cancer
- Affiliations
-
- 1Department of Gastroenterology, Sendai City Medical Center, Sendai, Japan. yoshi-hk@openhp.or.jp
- 2Natori Chuo Clinic, Sendai, Japan.
- 3Department of Gastroenterological Surgery, Sendai City Medical Center, Sendai, Japan.
- KMID: 2465802
- DOI: http://doi.org/10.5946/ce.2019.018
Abstract
- BACKGROUND/AIMS
In this study, we aimed to evaluate the predictive value of localized stenosis of the main pancreatic duct (MPD) for early detection of pancreatic cancer.
METHODS
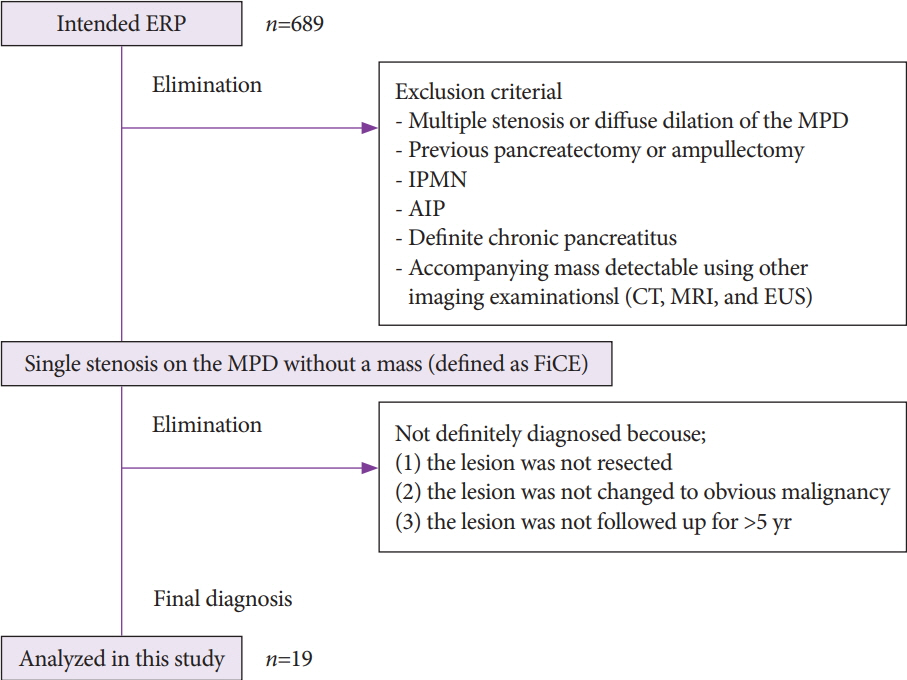
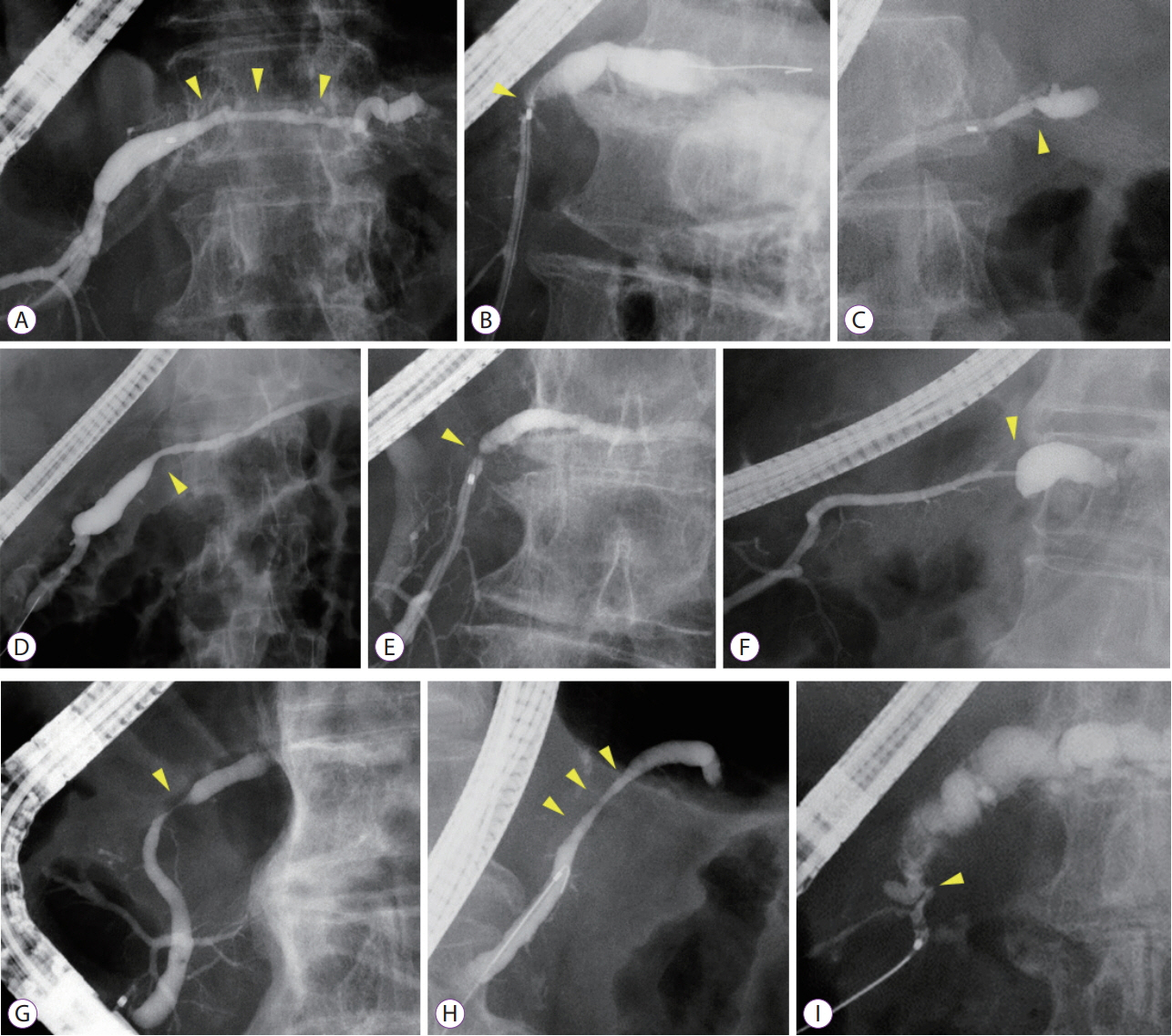
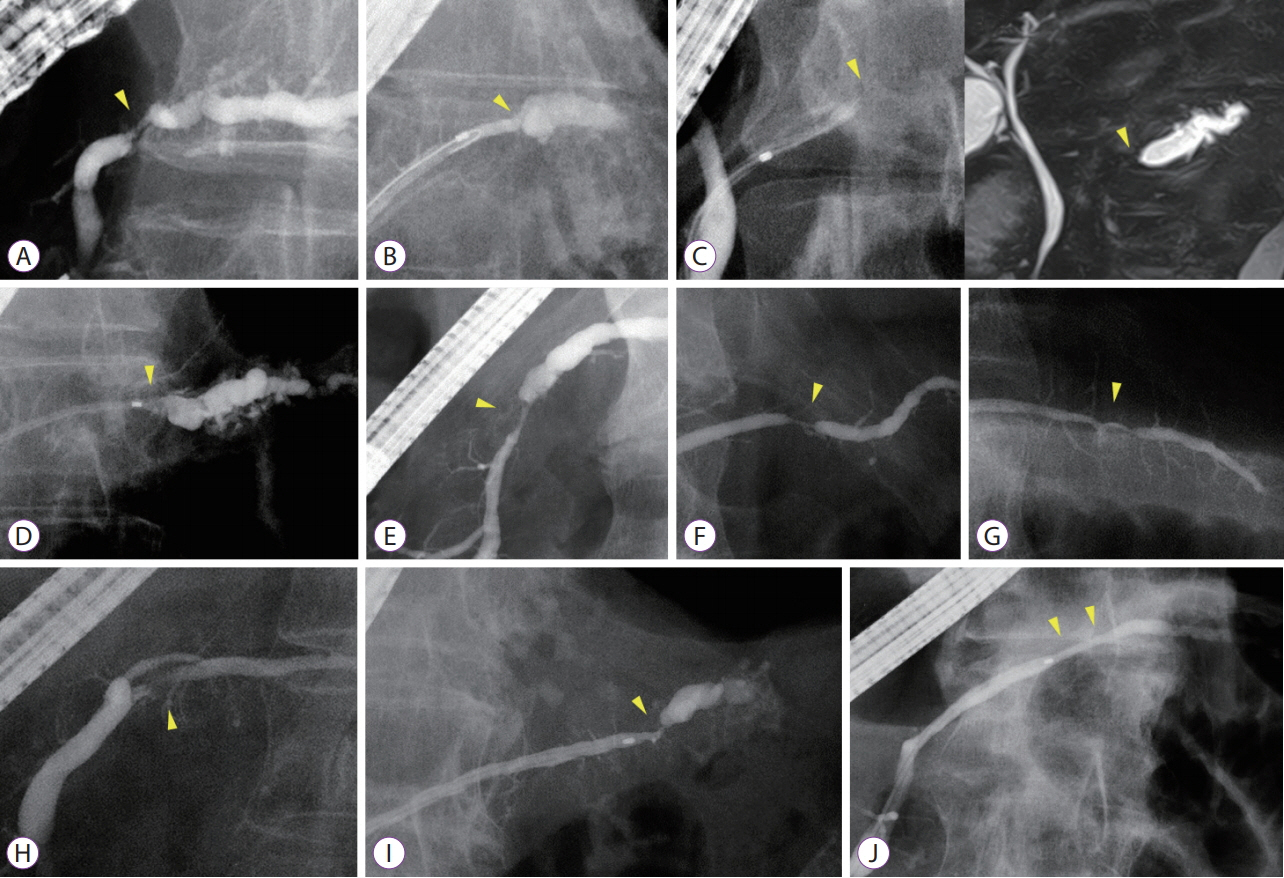
Among 689 patients who underwent endoscopic retrograde pancreatography from January 2008 to September 2018, 19 patients with MPD findings were enrolled. These patients showed findings for indicating suspicious pancreatic cancer at an early stage (FiCE); FiCE was defined as a single, localized stenosis in the MPD without a detectable mass (using any other imaging methods) and without other pancreatic diseases, such as definite chronic pancreatitis, intraductal papillary mucinous neoplasm, and autoimmune pancreatitis. Final diagnoses were established by examining resected specimens or through follow-up examinations after an interval of >5 years.
RESULTS
Among 19 patients with FiCE, 11 underwent surgical resection and 8 were evaluated after a >5-year observation period. The final diagnosis of the MPD stenosis was judged to be pancreatic cancer in 9 patients (47%), including 3 with intraepithelial cancer, and to be a non-neoplastic change in 10. The sensitivity, specificity, and accuracy of preoperative pancreatic juice cytology were 75%, 100%, and 88%, respectively.
CONCLUSIONS
The predictive value of FiCE for pancreatic cancer prevalence was 47%. Histological confirmation with pancreatic juice cytology is necessary before surgical resection.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Can Localized Stenosis of the Main Pancreatic Duct be a Predictive Factor for Early Detection of Pancreatic Cancer?
Mamoru Takenaka, Kentaro Yamao, Masatoshi Kudo
Clin Endosc. 2019;52(6):523-524. doi: 10.5946/ce.2019.204.
Reference
-
1. Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016; 388:73–85.
Article2. Kanno A, Masamune A, Hanada K, et al. Multicenter study of early pancreatic cancer in Japan. Pancreatology. 2018; 18:61–67.
Article3. Cancer Information Service. Cancer statistics in Japan; Table download [Internet]. Tokyo: National Cancer Center;c2018. [updated 2018 Mar 1; cited 2018 Nov 8]. Available from: https://ganjoho.jp/reg_stat/statistics/dl/index.html.4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018; 68:7–30.
Article5. Egawa S, Toma H, Ohigashi H, et al. Japan pancreatic cancer registry; 30th year anniversary: Japan Pancreas Society. Pancreas. 2012; 41:985–992.6. Hanada K, Okazaki A, Hirano N, et al. Diagnostic strategies for early pancreatic cancer. J Gastroenterol. 2015; 50:147–154.
Article7. Mouri T, Sasaki T, Serikawa M, et al. A comparison of 4-Fr with 5-Fr endoscopic nasopancreatic drainage catheters: a randomized, controlled trial. J Gastroenterol Hepatol. 2016; 31:1783–1789.
Article8. Hanada K, Amano H, Abe T. Early diagnosis of pancreatic cancer: current trends and concerns. Ann Gastroenterol Surg. 2017; 1:44–51.
Article9. Iiboshi T, Hanada K, Fukuda T, Yonehara S, Sasaki T, Chayama K. Value of cytodiagnosis using endoscopic nasopancreatic drainage for early diagnosis of pancreatic cancer: establishing a new method for the early detection of pancreatic carcinoma in situ. Pancreas. 2012; 41:523–529.10. Miyata T, Takenaka M, Omoto S, et al. A case of pancreatic carcinoma in situ diagnosed by repeated pancreatic juice cytology. Oncology. 2017; 93 Suppl 1:98–101.
Article11. Cotton PB, Lehman G, Vennes J, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991; 37:383–393.
Article12. Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010; 71:446–454.
Article13. Yokohata K, Shirahane K, Yonemasu H, et al. Focal ductal branch dilatation on magnetic resonance cholangiopancreatography: a hint for early diagnosis of pancreatic carcinoma. Scand J Gastroenterol. 2000; 35:1229–1232.14. Satoh T, Kikuyama M, Kawaguchi S, Kanemoto H, Muro H, Hanada K. Acute pancreatitis-onset carcinoma in situ of the pancreas with focal fat replacement diagnosed using serial pancreatic-juice aspiration cytologic examination (SPACE). Clin J Gastroenterol. 2017; 10:541–545.
Article15. Hanada K, Okazaki A, Hirano N, et al. Effective screening for early diagnosis of pancreatic cancer. Best Pract Res Clin Gastroenterol. 2015; 29:929–939.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Can Localized Stenosis of the Main Pancreatic Duct be a Predictive Factor for Early Detection of Pancreatic Cancer?
- Endoscopic retrograde cholangiopancreatography (ERCP) in pancreatic cancer
- A Case of Pancreatic Pseudocyst Resolved by Transpapillary Drainage via the Main Pancreatic Duct
- Endoscopic Ultrasound Can Differentiate High-Grade Pancreatic Intraepithelial Neoplasia, Small Pancreatic Ductal Adenocarcinoma, and Benign Stenosis
- Endoscopic Treatment of Main Pancreatic Duct Transection, Accompanied with Pseudocyst after Abdominal Trauma, with Using Pancreatic Duct Stent: A Case Report