J Pathol Transl Med.
2019 Nov;53(6):347-353. 10.4132/jptm.2019.09.29.
Interobserver Reproducibility of PD-L1 Biomarker in Non-small Cell Lung Cancer: A Multi-Institutional Study by 27 Pathologists
- Affiliations
-
- 1Department of Pathology, Inje University Ilsan Paik Hospital, Goyang, Korea.
- 2Department of Pathology, Konkuk University Medical Center, Konkuk University School of Medicine, Seoul, Korea.
- 3Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. ylachoi@skku.edu
- 4Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2465433
- DOI: http://doi.org/10.4132/jptm.2019.09.29
Abstract
- BACKGROUND
Assessment of programmed cell death-ligand 1 (PD-L1) immunohistochemical staining is used for treatment decisions in non-small cell lung cancer (NSCLC) regarding use of PD-L1/programmed cell death protein 1 (PD-1) immunotherapy. The reliability of the PD-L1 22C3 pharmDx assay is critical in guiding clinical practice. The Cardiopulmonary Pathology Study Group of the Korean Society of Pathologists investigated the interobserver reproducibility of PD-L1 staining with 22C3 pharmDx in NSCLC samples.
METHODS
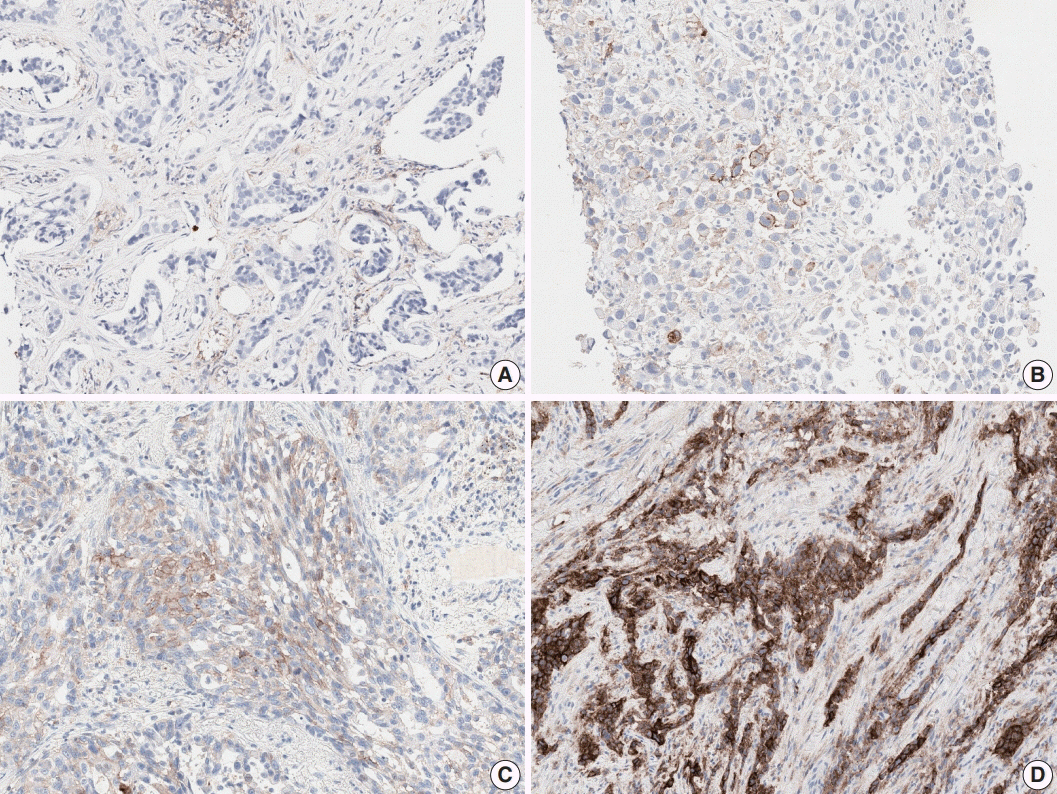
Twenty-seven pathologists individually assessed the tumor proportion score (TPS) for 107 NSCLC samples. Each case was divided into three levels based on TPS: <1%, 1%-49%, and ≥50%.
RESULTS
The intraclass correlation coefficient for TPS was 0.902±0.058. Weighted κ coefficient for 3-step assessment was 0.748±0.093. The κ coefficients for 1% and 50% cut-offs were 0.633 and 0.834, respectively. There was a significant association between interobserver reproducibility and experience (formal PD-L1 training, more experience for PD-L1 assessment, and longer practice duration on surgical pathology), histologic subtype, and specimen type.
CONCLUSIONS
Our results indicate that PD-L1 immunohistochemical staining provides a reproducible basis for decisions on anti-PD-1 therapy in NSCLC.
MeSH Terms
Figure
Cited by 1 articles
-
Programmed cell death-ligand 1 assessment in urothelial carcinoma: prospect and limitation
Kyu Sang Lee, Gheeyoung Choe
J Pathol Transl Med. 2021;55(3):163-170. doi: 10.4132/jptm.2021.02.22.
Reference
-
1. Guan J, Lim KS, Mekhail T, Chang CC. Programmed death ligand-1 (PD-L1) expression in the programmed death receptor-1 (PD-1)/PD-L1 blockade: a key player against various cancers. Arch Pathol Lab Med. 2017; 141:851–61.
Article2. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016; 375:1823–33.
Article3. Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016; 17:1497–508.
Article4. Gettinger S, Rizvi NA, Chow LQ, et al. Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. 2016; 34:2980–7.
Article5. Roach C, Zhang N, Corigliano E, et al. Development of a companion diagnostic PD-L1 immunohistochemistry assay for pembrolizumab therapy in non-small-cell lung cancer. Appl Immunohistochem Mol Morphol. 2016; 24:392–7.
Article6. Cree IA, Booton R, Cane P, et al. PD-L1 testing for lung cancer in the UK: recognizing the challenges for implementation. Histopathology. 2016; 69:177–86.7. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016; 387:1540–50.
Article8. Rimm DL, Han G, Taube JM, et al. A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non-small cell lung cancer. JAMA Oncol. 2017; 3:1051–8.
Article9. Cooper WA, Russell PA, Cherian M, et al. Intra- and interobserver reproducibility assessment of PD-L1 biomarker in non-small cell lung cancer. Clin Cancer Res. 2017; 23:4569–77.
Article10. Brunnström H, Johansson A, Westbom-Fremer S, et al. PD-L1 immunohistochemistry in clinical diagnostics of lung cancer: interpathologist variability is higher than assay variability. Mod Pathol. 2017; 30:1411–21.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- PD-L1 Testing in Non-small Cell Lung Cancer: Past, Present, and Future
- PD-L1 as a Biomarker in Gastric Cancer Immunotherapy
- Temporal evolution of programmed death-ligand 1 expression in patients with non-small cell lung cancer
- Interpretation of PD-L1 expression in gastric cancer: summary of a consensus meeting of Korean gastrointestinal pathologists
- Programmed Death Ligand 1 Immunohistochemistry in TripleNegative Breast Cancer: Evaluation of Inter-Pathologist Concordance and Inter-Assay Variability