J Pathol Transl Med.
2024 May;58(3):103-116. 10.4132/jptm.2024.03.15.
Interpretation of PD-L1 expression in gastric cancer: summary of a consensus meeting of Korean gastrointestinal pathologists
- Affiliations
-
- 1Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Department of Pathology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 3Seoul Clinical Laboratories, Department of Pathology, Yongin, Korea
- 4Department of Pathology, School of Medicine, Kyungpook National University, Kyungpook National University Chilgok Hospital, Daegu, Korea
- 5Department of Pathology, Yonsei University College of Medicine, Seoul, Korea
- 6Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 7Department of Pathology, Seoul National University Bundang Hospital, Seongnam, Korea
- 8Pathology Center, Seegene Medical Foundation, Seoul, Korea
- 9Department of Hospital Pathology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2555536
- DOI: http://doi.org/10.4132/jptm.2024.03.15
Abstract
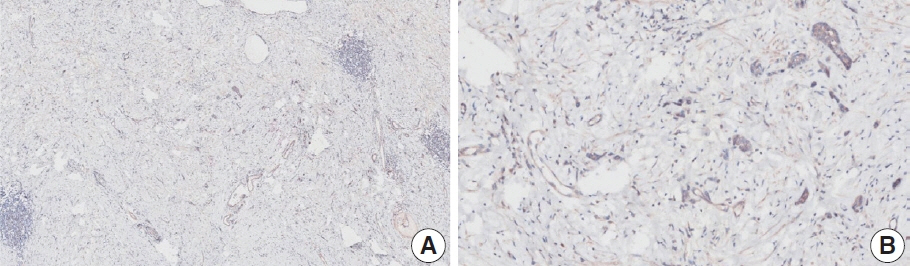
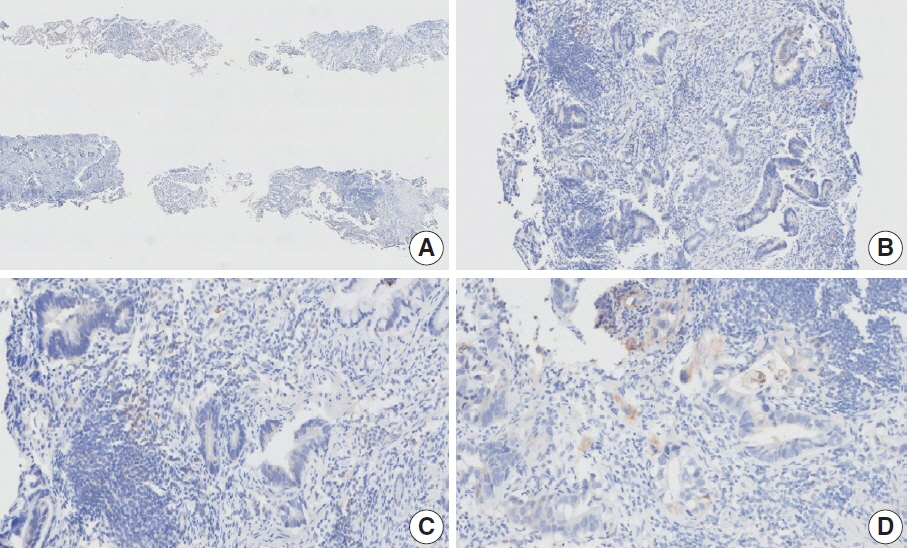
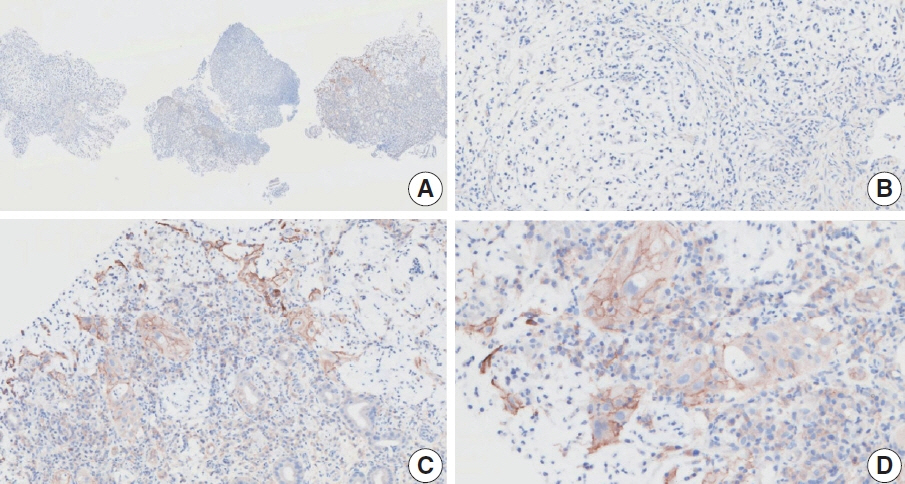
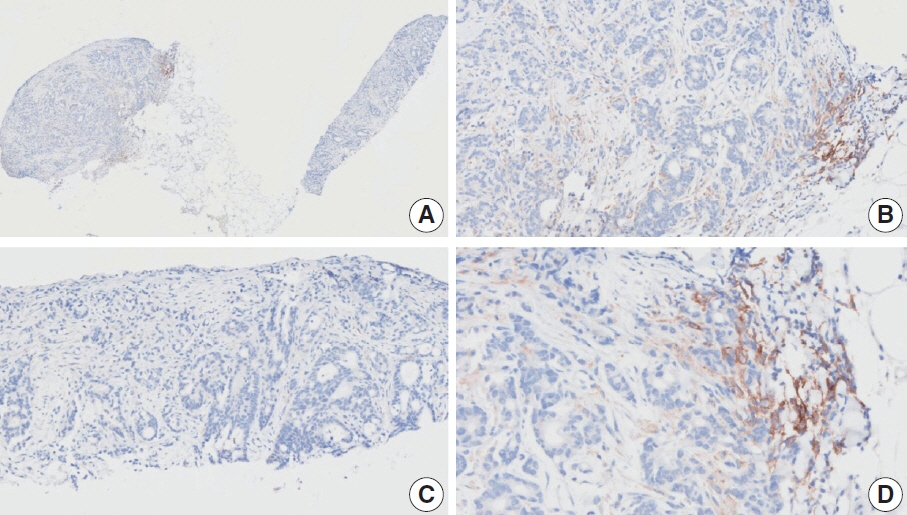
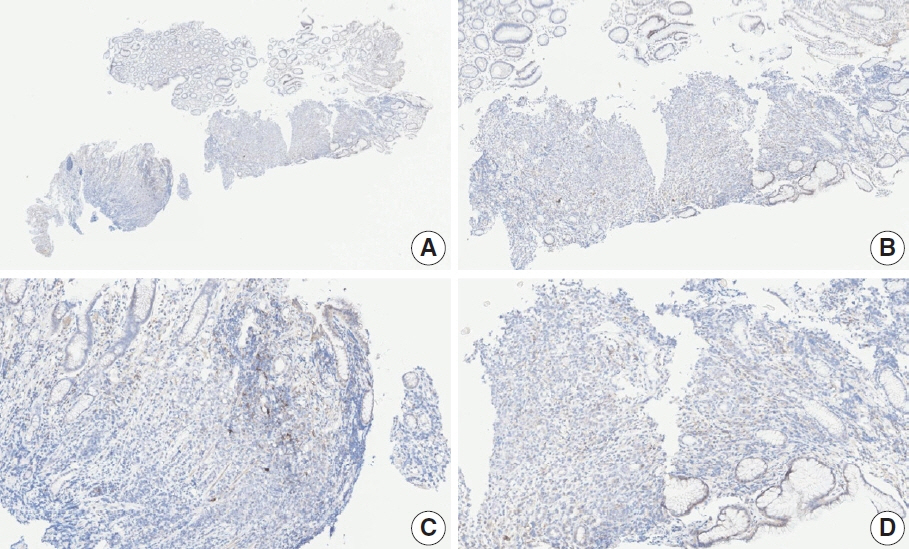
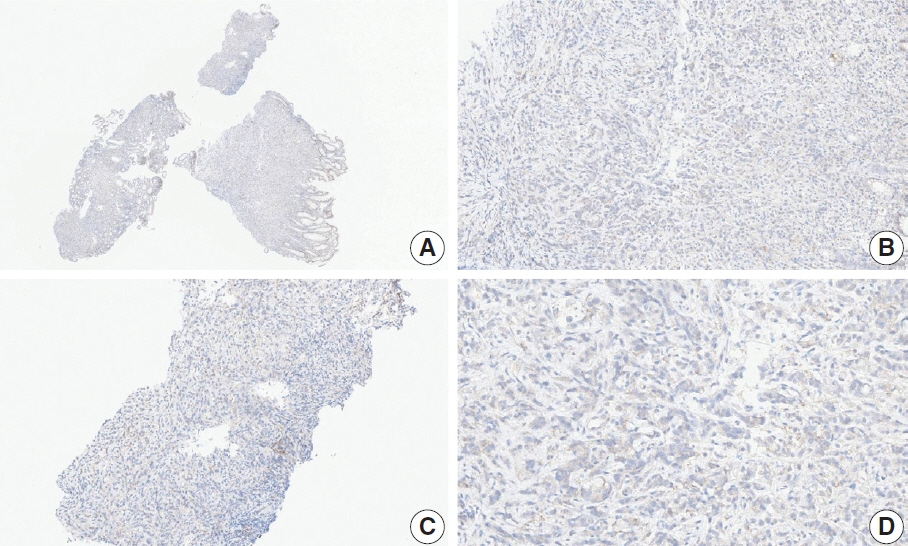
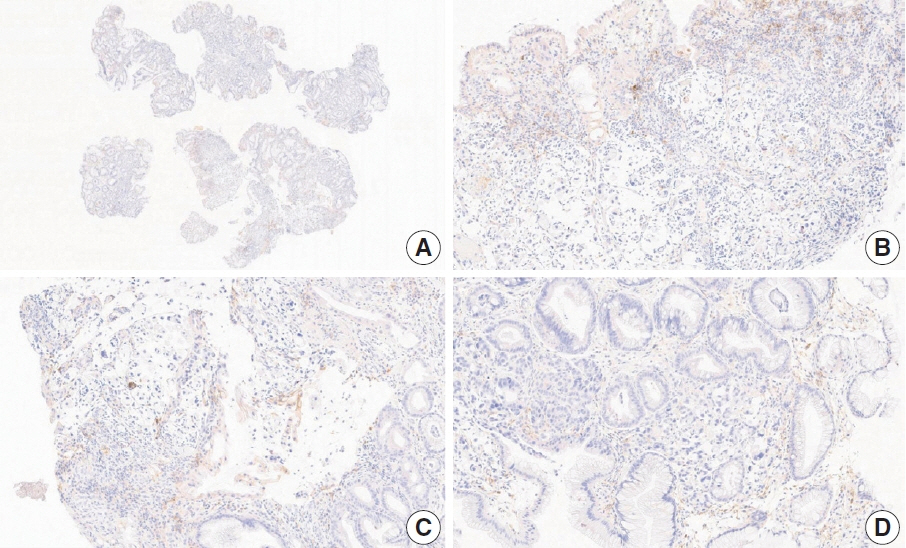
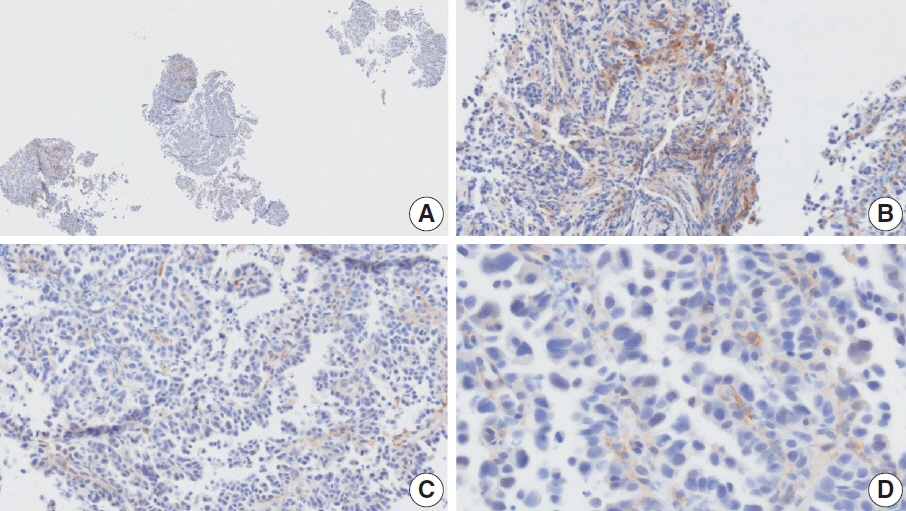
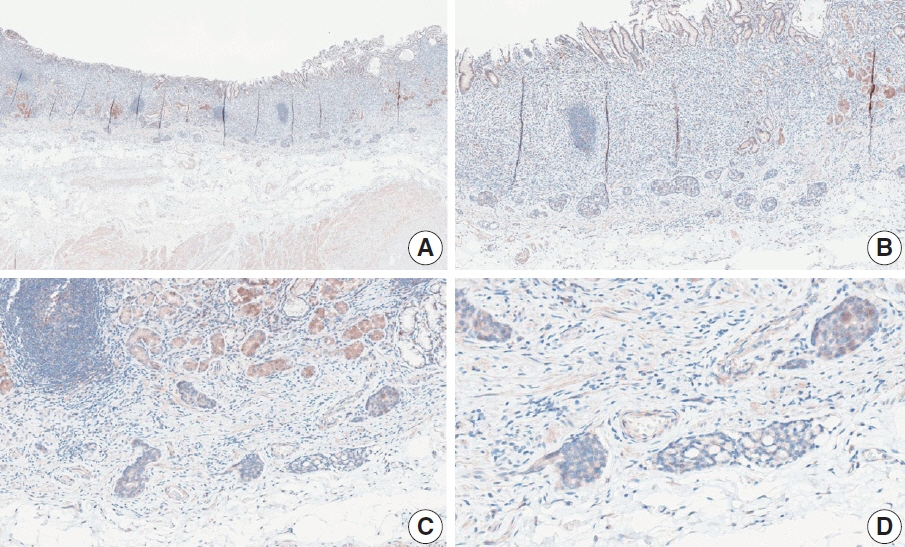
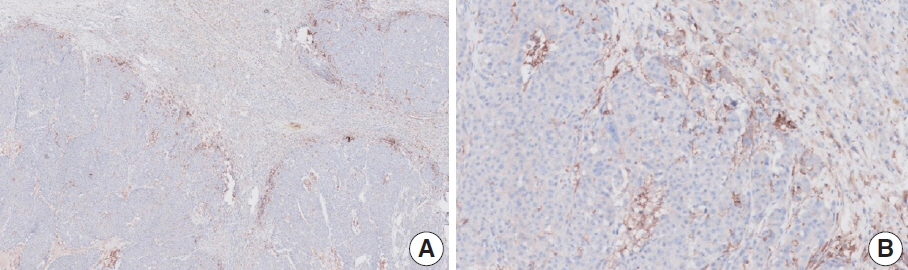
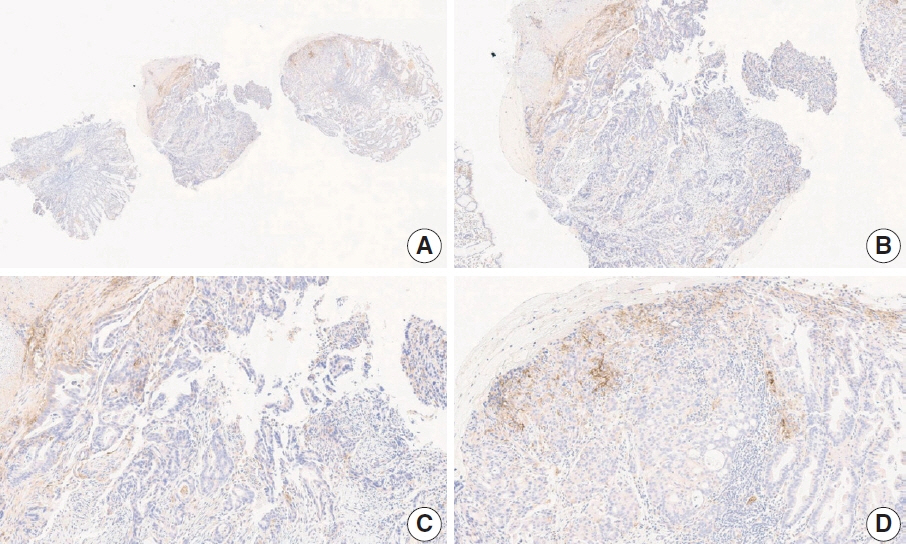
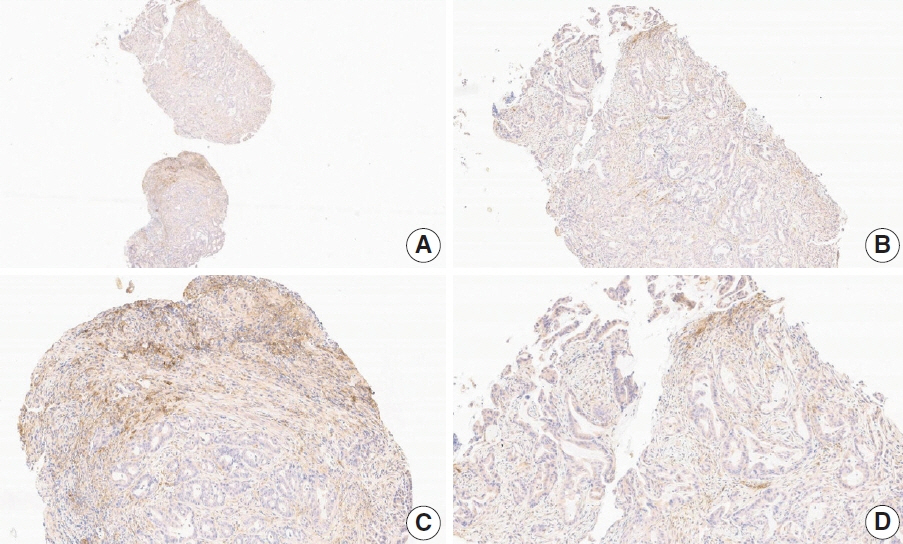
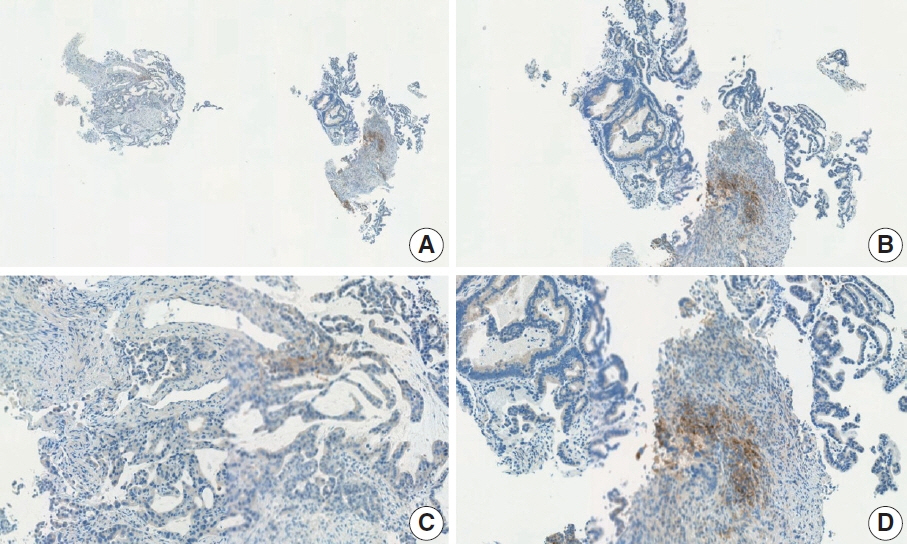
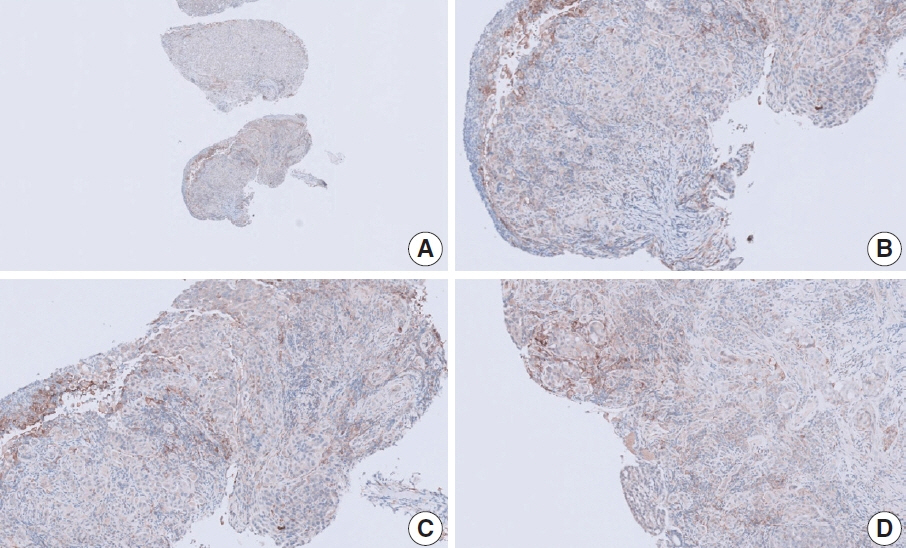
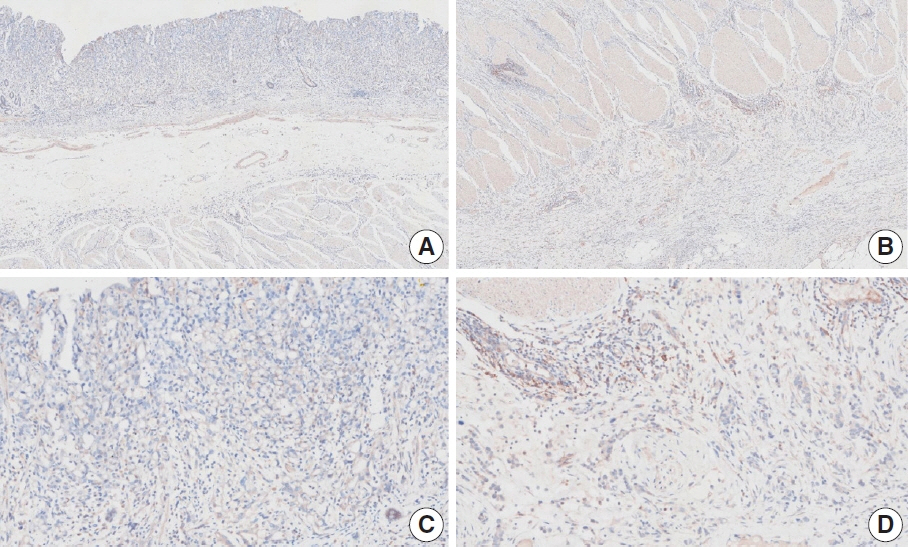
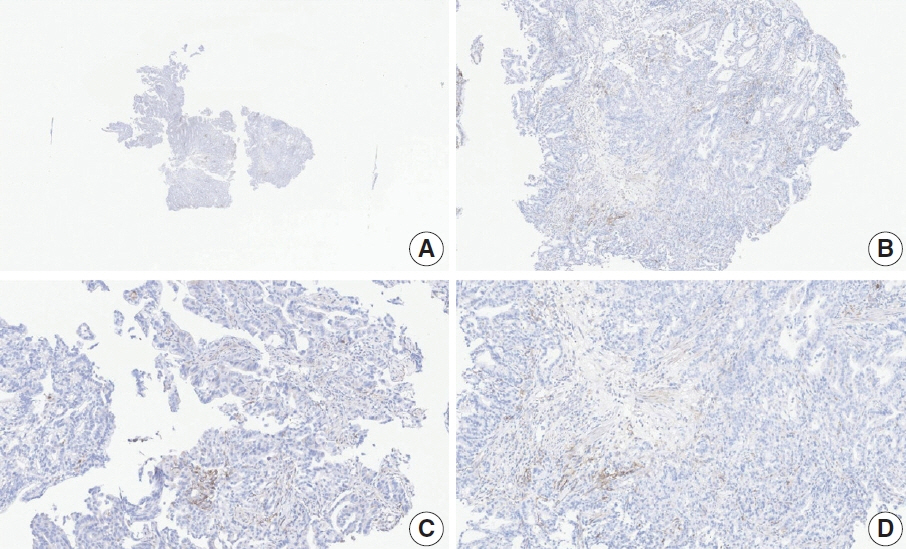
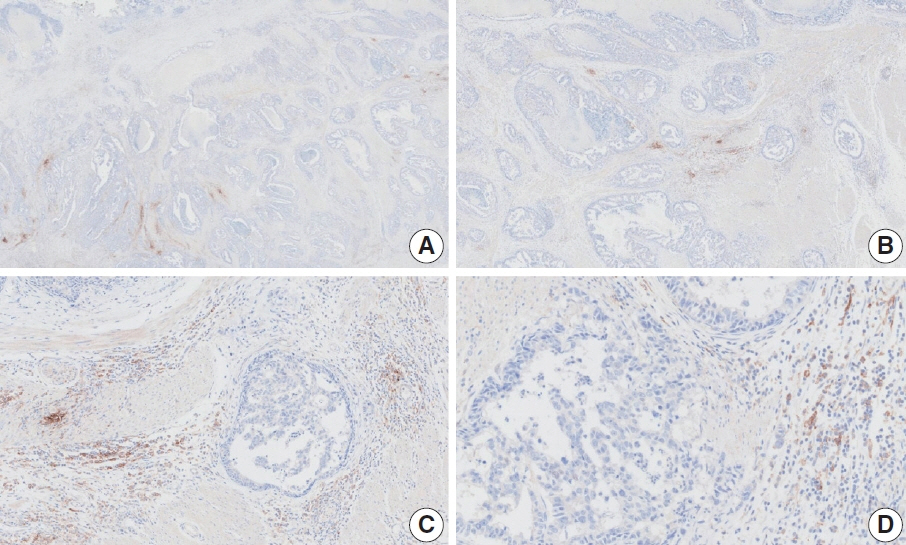
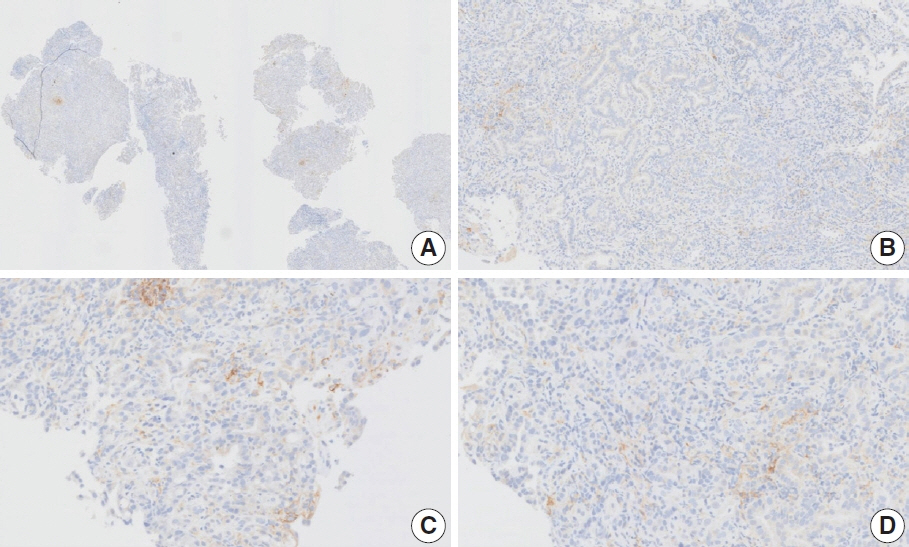
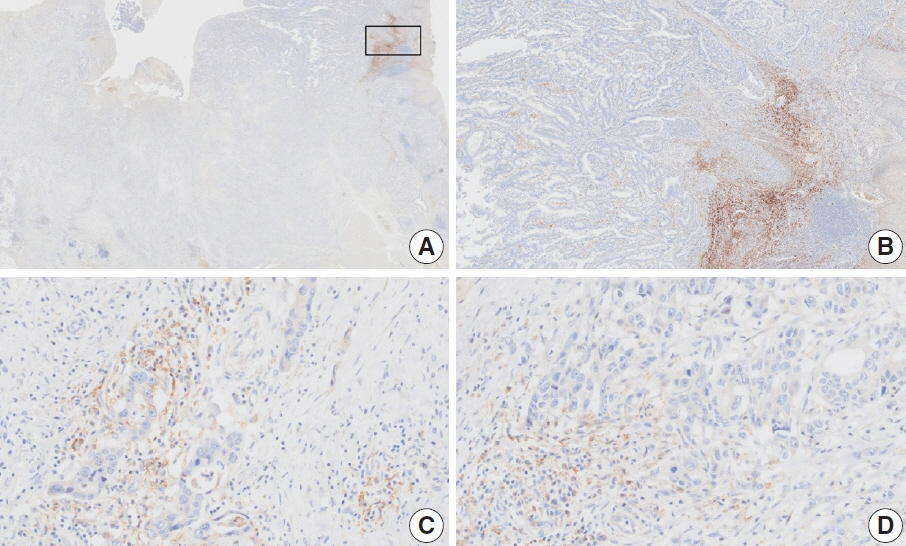
- Nivolumab plus chemotherapy in the first-line setting has demonstrated clinical efficacy in patients with human epidermal growth factor receptor 2–negative advanced or metastatic gastric cancer, and is currently indicated as a standard treatment. Programmed death-ligand 1 (PD-L1) expression is an important biomarker for predicting response to anti–programmed death 1/PD-L1 agents in several solid tumors, including gastric cancer. In the CheckMate-649 trial, significant clinical improvements were observed in patients with PD-L1 combined positive score (CPS) ≥ 5, determined using the 28-8 pharmDx assay. Accordingly, an accurate interpretation of PD-L1 CPS, especially at a cutoff of 5, is important. The CPS method evaluates both immune and tumor cells and provides a comprehensive assessment of PD-L1 expression in the tumor microenvironment of gastric cancer. However, CPS evaluation has several limitations, one of which is poor interobserver concordance among pathologists. Despite these limitations, clinical indications relying on PD-L1 CPS are increasing. In response, Korean gastrointestinal pathologists held a consensus meeting for the interpretation of PD-L1 CPS in gastric cancer. Eleven pathologists reviewed 20 PD-L1 slides with a CPS cutoff close to 5, stained with the 28-8 pharmDx assay, and determined the consensus scores. The issues observed in discrepant cases were discussed. In this review, we present cases of gastric cancer with consensus PD-L1 CPS. In addition, we briefly touch upon current practices and clinical issues associated with assays used for the assessment of PD-L1 expression in gastric cancer.
Keyword
Figure
Reference
-
References
1. Ribas A. Tumor immunotherapy directed at PD-1. N Engl J Med. 2012; 366:2517–9.
Article2. Nakamura Y, Kawazoe A, Lordick F, Janjigian YY, Shitara K. Biomarker-targeted therapies for advanced-stage gastric and gastrooesophageal junction cancers: an emerging paradigm. Nat Rev Clin Oncol. 2021; 18:473–87.
Article3. Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021; 398:27–40.
Article4. Kang YK, Chen LT, Ryu MH, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022; 23:234–47.
Article5. Rha SY, Oh DY, Yanez P, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): a multicentre, randomised, doubleblind, phase 3 trial. Lancet Oncol. 2023; 24:1181–95.6. Shitara K, Van Cutsem E, Bang YJ, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 2020; 6:1571–80.
Article7. Xu J, Kato K, Raymond E, et al. Tislelizumab plus chemotherapy versus placebo plus chemotherapy as first-line treatment for advanced or metastatic oesophageal squamous cell carcinoma (RATIONALE-306): a global, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2023; 24:483–95.
Article8. Janjigian YY, Kawazoe A, Bai Y, et al. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: interim analyses from the phase 3 KEYNOTE-811 randomised placebo-controlled trial. Lancet. 2023; 402:2197–208.
Article9. Shitara K, Ajani JA, Moehler M, et al. Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature. 2022; 603:942–8.
Article10. Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018; 4:e180013.11. Kubota Y, Aoki Y, Kawazoe A, Shitara K. Role of nivolumab in the management of first-Line unresectable advanced or recurrent gastric cancer in combination with chemotherapy: lessons from the Japanese experience. Cancer Manag Res. 2022; 14:3083–94.
Article12. Yi M, Jiao D, Xu H, et al. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol Cancer. 2018; 17:129.
Article13. Kulangara K, Zhang N, Corigliano E, et al. Clinical utility of the combined positive score for programmed death ligand-1 expression and the approval of pembrolizumab for treatment of gastric cancer. Arch Pathol Lab Med. 2019; 143:330–7.
Article14. Lei M, Siemers NO, Pandya D, et al. Analyses of PD-L1 and inflammatory gene expression association with efficacy of nivolumab +/- ipilimumab in gastric cancer/gastroesophageal junction cancer. Clin Cancer Res. 2021; 27:3926–35.
Article15. Park YS, Kook MC, Kim BH, et al. A standardized pathology report for gastric cancer: 2nd edition. J Pathol Transl Med. 2023; 57:1–27.
Article16. Kim TH, Kim IH, Kang SJ, et al. Korean Practice Guidelines for Gastric Cancer 2022: an evidence-based, multidisciplinary approach. J Gastric Cancer. 2023; 23:3–106.
Article17. Ahn S, Kim KM. PD-L1 expression in gastric cancer: interchangeability of 22C3 and 28-8 pharmDx assays for responses to immunotherapy. Mod Pathol. 2021; 34:1719–27.
Article18. Fernandez AI, Robbins CJ, Gaule P, et al. Multi-institutional study of pathologist reading of the programmed cell death ligand-1 combined positive score immunohistochemistry assay for gastric or gastroesophageal junction cancer. Mod Pathol. 2023; 36:100128.
Article19. Robert ME, Ruschoff J, Jasani B, et al. High interobserver variability among pathologists using combined positive score to evaluate PDL1 expression in gastric, gastroesophageal junction, and esophageal adenocarcinoma. Mod Pathol. 2023; 36:100154.
Article20. PD-L1 IHC 28-8 pharmDx interpretation manual: gastric adenocarcinoma, gastroesophageal juction (GEJ) adenocarcinoma, and esophageal adenocarcinoma [Internet]. Santa Clara: DAKO Agilent Technologies, 2021 [cited 2024 Feb 22]. Available from: https://www.agilent.com/cs/library/usermanuals/public/29456-d68866-pd-l1-28-8-gastric-interpretation-manual-en-eu.pdf.21. Nuti S, Zhang Y, Zerrouki N, et al. High interobserver and intraobserver reproducibility among pathologists assessing PD-L1 CPS across multiple indications. Histopathology. 2022; 81:732–41.
Article22. Park Y, Koh J, Na HY, et al. PD-L1 testing in gastric cancer by the combined positive score of the 22C3 PharmDx and SP263 assay with clinically relevant cut-offs. Cancer Res Treat. 2020; 52:661–70.
Article23. Tsao MS, Kerr KM, Kockx M, et al. PD-L1 immunohistochemistry comparability study in real-life clinical samples: results of Blueprint Phase 2 Project. J Thorac Oncol. 2018; 13:1302–11.
Article24. Prince EA, Sanzari JK, Pandya D, Huron D, Edwards R. Analytical concordance of PD-L1 assays utilizing antibodies from FDA-approved diagnostics in advanced cancers: a systematic literature review. JCO Precis Oncol. 2021; 5:953–73.
Article25. Akhtar M, Rashid S, Al-Bozom IA. PD-L1 immunostaining: what pathologists need to know. Diagn Pathol. 2021; 16:94.
Article26. O’Malley DP, Yang Y, Boisot S, et al. Immunohistochemical detection of PD-L1 among diverse human neoplasms in a reference laboratory: observations based upon 62,896 cases. Mod Pathol. 2019; 32:929–42.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- PD-L1 as a Biomarker in Gastric Cancer Immunotherapy
- The Expression of Programmed Death-Ligand 1 on Immune Cells Is Related to a Better Prognosis in Biliary Tract Cancer
- PD-L1 Testing in Non-small Cell Lung Cancer: Past, Present, and Future
- Prognostic Perspectives of STING and PD-L1 Expression and Correlation with the Prognosis of Epstein-Barr Virus-Associated Gastric Cancers
- Immunohistochemical expression of programmed death-ligand 1 and CD8 in glioblastomas