J Korean Soc Radiol.
2019 Nov;80(6):1160-1178. 10.3348/jksr.2019.80.6.1160.
Neural Mechanism of Second Language Processing in Korean-English Bilingual Children
- Affiliations
-
- 1Department of Radiology, Chonnam National University Medical School, Gwangju, Korea. radyoon@jnu.ac.kr
- 2Department of Radiology, Chonnam National University Hwasun Hospital, Hwasun, Korea.
- 3Department of English Linguistics and Literature, College of Humanity, Chonnam National University, Gwangju, Korea.
- KMID: 2464914
- DOI: http://doi.org/10.3348/jksr.2019.80.6.1160
Abstract
- PURPOSE
To evaluate the neural mechanism of second language processing in Korean-English bilingual children using functional MRI (fMRI).
MATERIALS AND METHODS
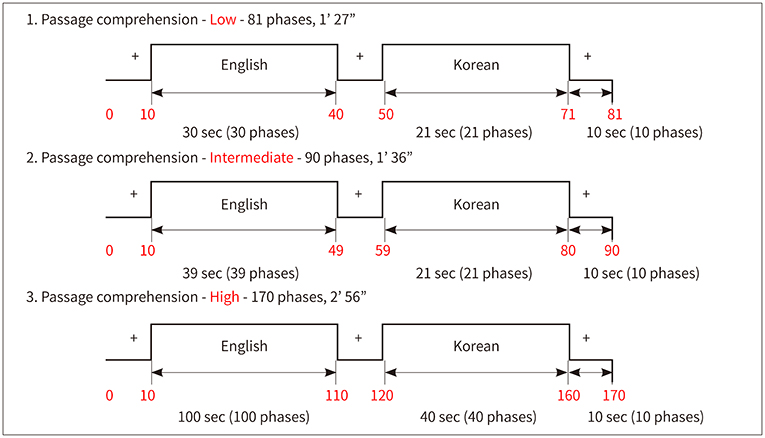
The study was conducted on 20 Korean elementary school children who were learning English as a foreign language. fMRI was performed during short-passage comprehension tasks in Korean and English languages. We analyzed which brain areas were activated according to the language, English proficiency, and task difficulty.
RESULTS
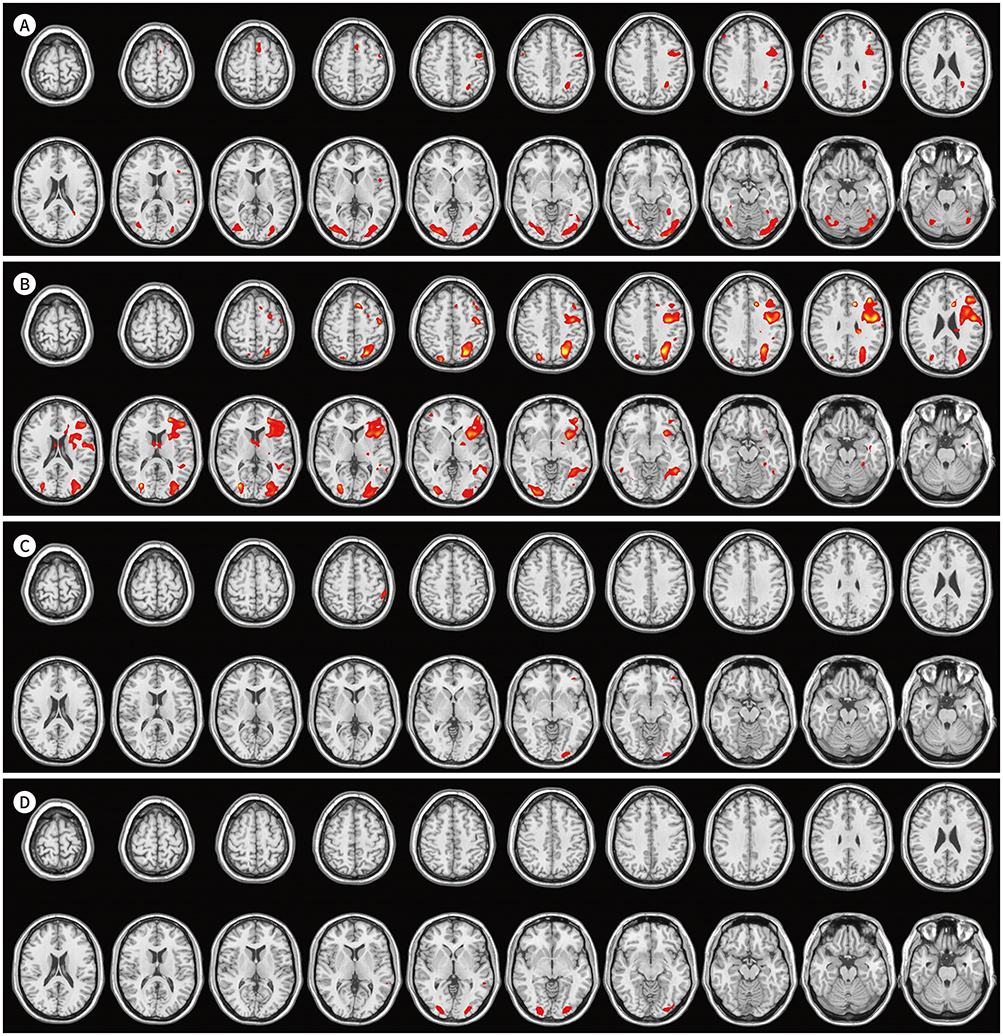
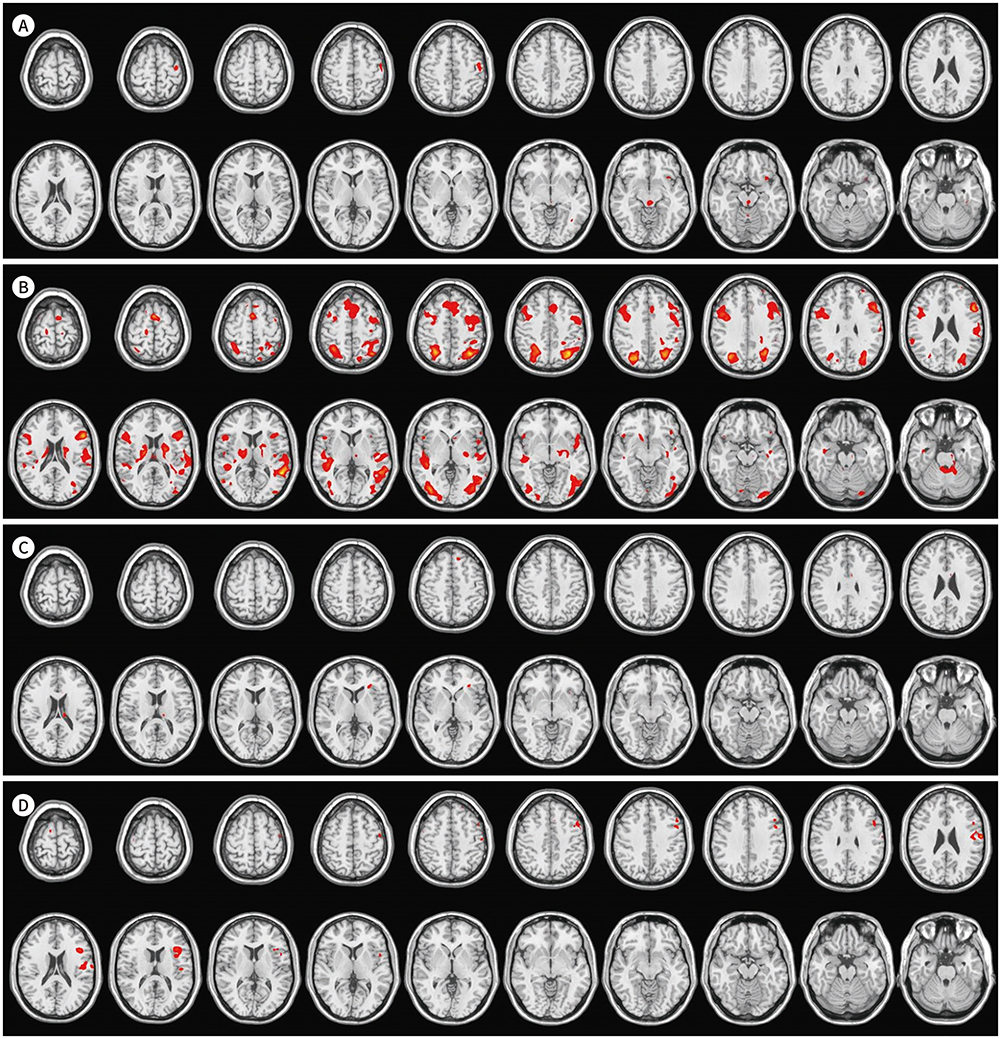
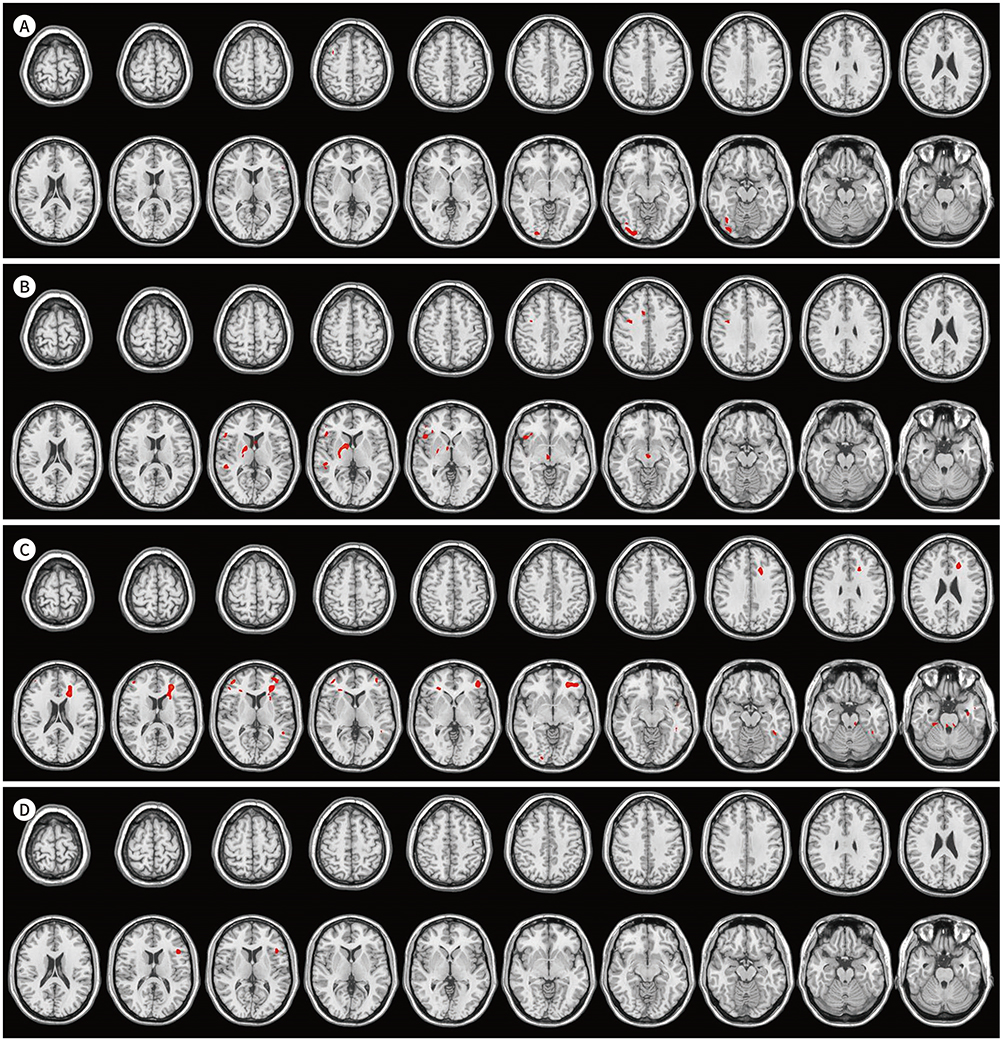
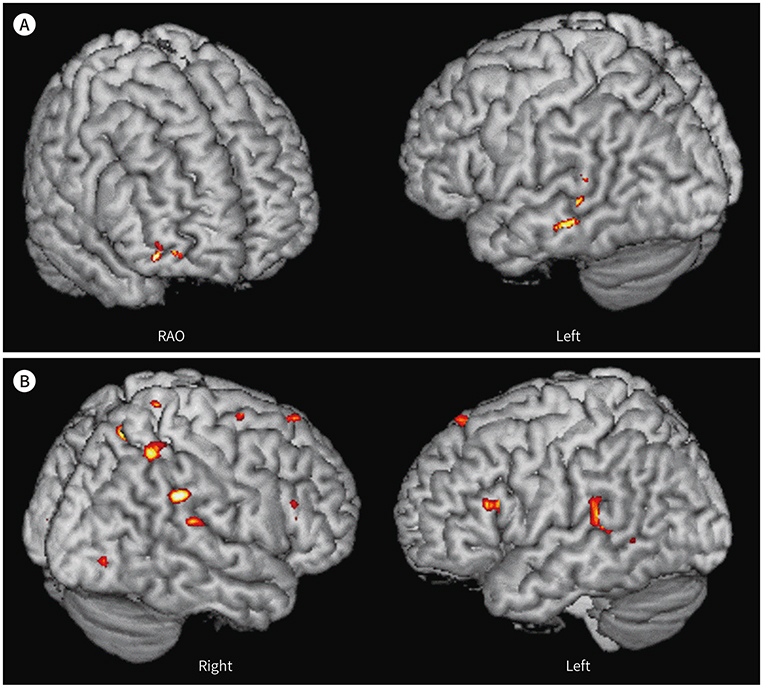
Higher activities were observed in the dorsolateral prefrontal cortex, supplementary motor area, precentral gyrus, left basal ganglia, and left temporoparietal and occipital lobes during English comprehension than during Korean comprehension. The low English proficiency group showed higher activities than the high English proficiency group in the frontotemporal cortex, including the prefrontal cortex. Higher activities were observed in the right inferior frontal gyrus and right temporoparietal lobe during the English comprehension task of intermediate difficulty compared to that of low difficulty. However, the brain activities significantly decreased while performing a high-difficulty English task.
CONCLUSION
Brain activities significantly increased during English comprehension in the lower English proficiency group while performing an intermediate-difficulty task. However, brain activation decreased when the task difficulty exceeded the moderate comprehension level. These results suggest that a proper level of education is important to learn a second language.
MeSH Terms
Figure
Reference
-
1. European Commission. Special Eurobarometer 386. Europeans and their languages. Accessed Nov 5, 2018. Available at. http://ec.europa.eu/public_opinion/index_en.htm.2. Lenneberg EH. The biological foundations of language. Hosp Pract. 1967; 2:59–67.3. Johnson JS, Newport EL. Critical period effects in second language learning: the influence of maturational state on the acquisition of English as a second language. Cogn Psychol. 1989; 21:60–99.4. Korean Society of Magnetic Resonance in Medicine. Magnetic resonance imaging. Seoul: Ilchokak;2008.5. Chee MW, Caplan D, Soon CS, Sriram N, Tan EW, Thiel T, et al. Processing of visually presented sentences in Mandarin and English studied with fMRI. Neuron. 1999; 23:127–137.6. Illes J, Francis WS, Desmond JE, Gabrieli JD, Glover GH, Poldrack R, et al. Convergent cortical representation of semantic processing in bilinguals. Brain Lang. 1999; 70:347–363.7. Hernandez AE, Martinez A, Kohnert K. In search of the language switch: an fMRI study of picture naming in Spanish-English bilinguals. Brain Lang. 2000; 73:421–431.8. Hernandez AE, Dapretto M, Mazziotta J, Bookheimer S. Language switching and language representation in Spanish-English bilinguals: an fMRI study. Neuroimage. 2001; 14:510–520.9. Kim KH, Relkin NR, Lee KM, Hirsch J. Distinct cortical areas associated with native and second languages. Nature. 1997; 388:171–174.10. Marian V, Spivey M, Hirsch J. Shared and separate systems in bilingual language processing: converging evidence from eyetracking and brain imaging. Brain Lang. 2003; 86:70–82.11. Wartenburger I, Heekeren HR, Abutalebi J, Cappa SF, Villringer A, Perani D. Early setting of grammatical processing in the bilingual brain. Neuron. 2003; 37:159–170.12. Hull R, Vaid J. Bilingual language lateralization: a meta-analytic tale of two hemispheres. Neuropsychologia. 2007; 45:1987–2008.13. Cho JM, Ryoo JW, Choi DS, Shin TB, Chung SH, Kim JE, et al. Functional MRI of multilingual subjects. J Korean Soc Radiol. 2009; 61:351–358.14. Woodcock RW, Mather N, Schrank FA. Woodcock-Johnson III diagnostic reading battery. Itasca: Riverside Publishing Company;2004.15. Abutalebi J, Green DW. Control mechanisms in bilingual language production: neural evidence from language switching studies. Lang Cogn Process. 2008; 23:557–582.16. Rodriguez-Fornells A, De Diego Balaguer R, Münte TF. Executive control in bilingual language processing. Lang Learn. 2006; 56:133–190.17. Braun AR, Guillemin A, Hosey L, Varga M. The neural organization of discourse: an H2 15O-PET study of narrative production in English and American sign language. Brain. 2001; 124:2028–2044.18. Wise RJ, Greene J, Büchel C, Scott SK. Brain regions involved in articulation. Lancet. 1999; 353:1057–1061.19. Booth JR, Wood L, Lu D, Houk JC, Bitan T. The role of the basal ganglia and cerebellum in language processing. Brain Res. 2007; 1133:136–144.20. Poeppel D, Hickok G. Towards a new functional anatomy of language. Cognition. 2004; 92:1–12.21. Friederici AD. Towards a neural basis of auditory sentence processing. Trends Cogn Sci. 2002; 6:78–84.22. Keller TA, Carpenter PA, Just MA. The neural bases of sentence comprehension: a fMRI examination of syntactic and lexical processing. Cereb Cortex. 2001; 11:223–237.23. Sakai KL, Miura K, Narafu N, Muraishi Y. Correlated functional changes of the prefrontal cortex in twins induced by classroom education of second language. Cereb Cortex. 2004; 14:1233–1239.24. Chee MW, Hon N, Lee HL, Soon CS. Relative language proficiency modulates BOLD signal change when bilinguals perform semantic judgments. Blood oxygen level dependent. Neuroimage. 2001; 13:1155–1116.25. Hernandez AE. The bilingual brain. Oxford: Oxford University Press;2013.26. Davey J, Thompson HE, Hallam G, Karapanagiotidis T, Murphy C, De Caso I, et al. Exploring the role of the posterior middle temporal gyrus in semantic cognition: integration of anterior temporal lobe with executive processes. Neuroimage. 2016; 137:165–177.27. Noonan KA, Jefferies E, Visser M, Lambon Ralph MA. Going beyond inferior prefrontal involvement in semantic control: evidence for the additional contribution of dorsal angular gyrus and posterior middle temporal cortex. J Cogn Neurosci. 2013; 25:1824–1850.28. Just MA, Carpenter PA, Keller TA, Eddy WF, Thulborn KR. Brain activation modulated by sentence comprehension. Science. 1996; 274:114–116.29. Carpenter PA, Just MA, Keller TA, Eddy W, Thulborn K. Graded functional activation in the visuospatial system with the amount of task demand. J Cogn Neurosci. 1999; 11:9–24.30. Grasby PM, Frith CD, Friston KJ, Simpson J, Fletcher PC, Frackowiak RS, et al. A graded task approach to the functional mapping of brain areas implicated in auditory-verbal memory. Brain. 1994; 117:1271–1282.31. Rypma B, Prabhakaran V, Desmond JE, Glover GH, Gabrieli JD. Load-dependent roles of frontal brain regions in the maintenance of working memory. Neuroimage. 1999; 9:216–226.32. Fridriksson J, Morrow L. Cortical activation and language task difficulty in aphasia. Aphasiology. 2005; 19:239–250.33. Hasegawa M, Carpenter PA, Just MA. An fMRI study of bilingual sentence comprehension and workload. Neuroimage. 2002; 15:647–660.34. Brodtmann A, Puce A, Darby D, Donnan G. Regional fMRI brain activation does correlate with global brain volume. Brain Res. 2009; 1259:17–25.35. Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, et al. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci U S A. 2000; 97:4398–4403.36. Narr KL, Woods RP, Thompson PM, Szeszko P, Robinson D, Dimtcheva T, et al. Relationships between IQ and regional cortical gray matter thickness in healthy adults. Cereb Cortex. 2007; 17:2163–2171.37. Manard M, Bahri MA, Salmon E, Collette F. Relationship between grey matter integrity and executive abilities in aging. Brain Res. 2016; 1642:562–580.38. Takeuchi H, Taki Y, Nouchi R, Yokoyama R, Kotozaki Y, Nakagawa S, et al. Global associations between regional gray matter volume and diverse complex cognitive functions: evidence from a large sample study. Sci Rep. 2017; 7:10014.39. Kharitonova M, Martin RE, Gabrieli JD, Sheridan MA. Cortical gray-matter thinning is associated with age-related improvements on executive function tasks. Dev Cogn Neurosci. 2013; 6:61–71.40. Caplan R, Levitt J, Siddarth P, Wu KN, Gurbani S, Shields WD, et al. Language and brain volumes in children with epilepsy. Epilepsy Behav. 2010; 17:402–407.41. Narr KL, Woods RP, Lin J, Kim J, Phillips OR, Del'Homme M, et al. Widespread cortical thinning is a robust anatomical marker for attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2009; 48:1014–1022.42. Batty MJ, Liddle EB, Pitiot A, Toro R, Groom MJ, Scerif G, et al. Cortical gray matter in attention-deficit/hyperactivity disorder: a structural magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry. 2010; 49:229–238.43. Joo EY, Tae WS, Lee MJ, Kang JW, Park HS, Lee JY, et al. Reduced brain gray matter concentration in patients with obstructive sleep apnea syndrome. Sleep. 2010; 33:235–241.44. Rogers JC, De Brito SA. Cortical and subcortical gray matter volume in youths with conduct problems: a meta-analysis. JAMA Psychiatry. 2016; 73:64–72.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Relation between Phonological Processing, Auditory Processing and Speech Perception among Bilingual Poor Readers
- Information Processing Characteristics of the Patients with Specific Language Impairment Using Kaufman-Assessment Battery for Children
- A De-identification Method for Bilingual Clinical Texts of Various Note Types
- O-JMeSH: creating a bilingual English-Japanese controlled vocabulary of MeSH UIDs through machine translation and mutual information
- open-japanese-mesh: assigning MeSH UIDs to Japanese medical terms via open Japanese-English glossaries