Korean Circ J.
2019 Dec;49(12):1115-1122. 10.4070/kcj.2019.0211.
Understanding Vulnerable Plaques: Current Status and Future Directions
- Affiliations
-
- 1Cardiovascular Center and Cardiology Division, Incheon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 2Cardiovascular Center and Cardiology Division, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. kiyuk@catholic.ac.kr
- KMID: 2464291
- DOI: http://doi.org/10.4070/kcj.2019.0211
Abstract
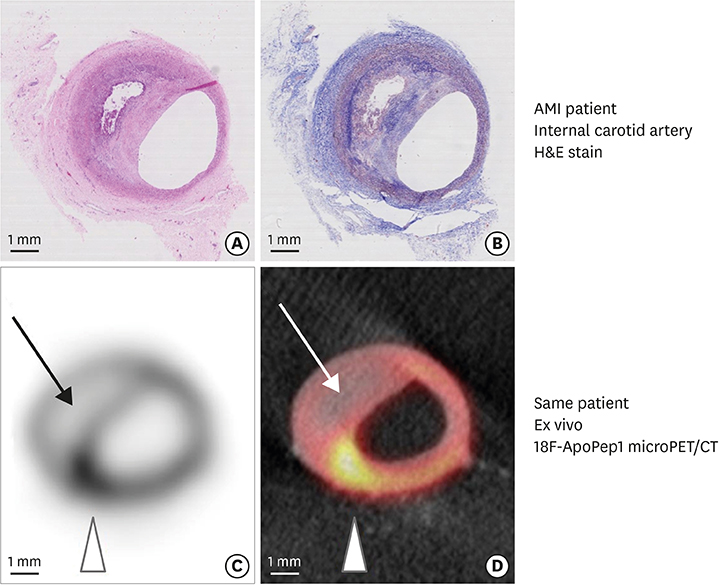
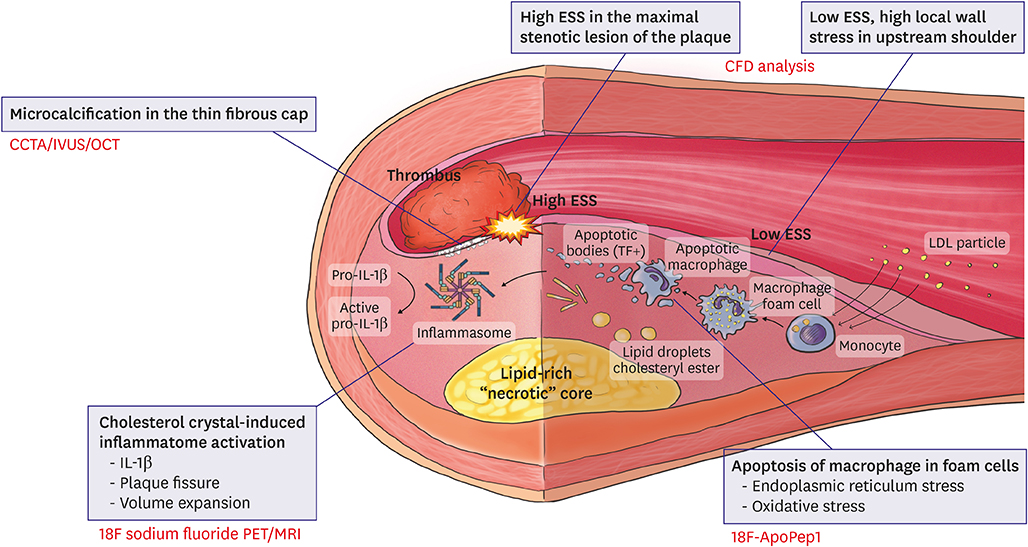
- The main cause of acute myocardial infarction is plaque rupture accompanied by superimposed coronary thrombosis. Thin-cap fibroatheromas (TCFAs) have been suggested as a type of lesion with a vulnerability that can cause plaque rupture. However, not only the existence of a TCFA but also the fine and complex interactions of other anatomical and hemodynamic factors, such as microcalcification in the fibrous cap, cholesterol crystal-induced inflammasome activation, the apoptosis of intraplaque macrophages, and endothelial shear stress distribution should precede a clinical event caused by plaque rupture. Recent studies are being conducted to identify these mechanisms through molecular imaging and hemodynamic assessment using computational fluid dynamics, which will result in better clinical results through selective coronary interventions.
MeSH Terms
Figure
Cited by 2 articles
-
Role of Inflammation in Arterial Calcification
Hae-Young Lee, Soyeon Lim, Sungha Park
Korean Circ J. 2021;51(2):114-125. doi: 10.4070/kcj.2020.0517.Coronary Physiology-Based Approaches for Plaque Vulnerability: Implications for Risk Prediction and Treatment Strategies
Seokhun Yang, Bon-Kwon Koo
Korean Circ J. 2023;53(9):581-593. doi: 10.4070/kcj.2023.0117.
Reference
-
1. Schuurman AS, Vroegindewey MM, Kardys I, et al. Prognostic value of intravascular ultrasound in patients with coronary artery disease. J Am Coll Cardiol. 2018; 72:2003–2011.2. Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on coronary plaque composition. J Am Coll Cardiol. 2018; 72:2012–2021.3. Arbab-Zadeh A, Fuster V. The myth of the “vulnerable plaque”: transitioning from a focus on individual lesions to atherosclerotic disease burden for coronary artery disease risk assessment. J Am Coll Cardiol. 2015; 65:846–855.4. Tian J, Ren X, Vergallo R, et al. Distinct morphological features of ruptured culprit plaque for acute coronary events compared to those with silent rupture and thin-cap fibroatheroma: a combined optical coherence tomography and intravascular ultrasound study. J Am Coll Cardiol. 2014; 63:2209–2216.5. Toutouzas K, Karanasos A, Tsiamis E, et al. New insights by optical coherence tomography into the differences and similarities of culprit ruptured plaque morphology in non-ST-elevation myocardial infarction and ST-elevation myocardial infarction. Am Heart J. 2011; 161:1192–1199.6. deFilippi CR, de Lemos JA, Tkaczuk AT, et al. Physical activity, change in biomarkers of myocardial stress and injury, and subsequent heart failure risk in older adults. J Am Coll Cardiol. 2012; 60:2539–2547.7. Otsuka F, Sakakura K, Yahagi K, Joner M, Virmani R. Has our understanding of calcification in human coronary atherosclerosis progressed? Arterioscler Thromb Vasc Biol. 2014; 34:724–736.8. Vedre A, Pathak DR, Crimp M, Lum C, Koochesfahani M, Abela GS. Physical factors that trigger cholesterol crystallization leading to plaque rupture. Atherosclerosis. 2009; 203:89–96.9. Kietselaer BL, Reutelingsperger CP, Heidendal GA, et al. Noninvasive detection of plaque instability with use of radiolabeled annexin A5 in patients with carotid-artery atherosclerosis. N Engl J Med. 2004; 350:1472–1473.10. Pedrigi RM, de Silva R, Bovens SM, Mehta VV, Petretto E, Krams R. Thin-cap fibroatheroma rupture is associated with a fine interplay of shear and wall stress. Arterioscler Thromb Vasc Biol. 2014; 34:2224–2231.11. Herrick JB. Landmark article (JAMA 1912). Clinical features of sudden obstruction of the coronary arteries. By James B. Herrick. JAMA. 1983; 250:1757–1765.12. Clark E, Graef I, Chasis H. Thrombosis of the aorta and coronary arteries with specific reference to the “fibrinoid” lesions. Arch Pathol (Chic). 1936; 22:183–212.13. Constantinides P. Plaque fissures in human coronary thrombosis. Fed Prox. 1964; 23:443.14. Davies MJ. Stability and instability: two faces of coronary atherosclerosis. The Paul Dudley White Lecture 1995. Circulation. 1996; 94:2013–2020.15. Jenniskens M, Langouche L, Van den Berghe G. Cholestatic alterations in the critically ill: some new light on an old problem. Chest. 2018; 153:733–743.16. Schaar JA, Muller JE, Falk E, et al. Terminology for high-risk and vulnerable coronary artery plaques. Report of a meeting on the vulnerable plaque, June 17 and 18, 2003, Santorini, Greece. Eur Heart J. 2004; 25:1077–1082.17. Kolodgie FD, Burke AP, Farb A, et al. The thin-cap fibroatheroma: a type of vulnerable plaque: the major precursor lesion to acute coronary syndromes. Curr Opin Cardiol. 2001; 16:285–292.18. Kolodgie FD, Virmani R, Burke AP, et al. Pathologic assessment of the vulnerable human coronary plaque. Heart. 2004; 90:1385–1391.19. Beckman JA, Ganz J, Creager MA, Ganz P, Kinlay S. Relationship of clinical presentation and calcification of culprit coronary artery stenoses. Arterioscler Thromb Vasc Biol. 2001; 21:1618–1622.20. Karanasos A, Ligthart JM, Witberg KT, Regar E. Calcified nodules: an underrated mechanism of coronary thrombosis? JACC Cardiovasc Imaging. 2012; 5:1071–1072.21. Ehara S, Kobayashi Y, Yoshiyama M, et al. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation. 2004; 110:3424–3429.22. Motoyama S, Kondo T, Sarai M, et al. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol. 2007; 50:319–326.23. Kivimäki M, Pentti J, Ferrie JE, et al. Work stress and risk of death in men and women with and without cardiometabolic disease: a multicohort study. Lancet Diabetes Endocrinol. 2018; 6:705–713.24. Vengrenyuk Y, Carlier S, Xanthos S, et al. A hypothesis for vulnerable plaque rupture due to stress-induced debonding around cellular microcalcifications in thin fibrous caps. Proc Natl Acad Sci U S A. 2006; 103:14678–14683.25. Kashiwagi M, Liu L, Chu KK, et al. Feasibility of the assessment of cholesterol crystals in human macrophages using micro optical coherence tomography. PLoS One. 2014; 9:e102669.26. Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017; 377:1119–1131.27. Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010; 10:36–46.28. Tabas I. The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ Res. 2010; 107:839–850.29. Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011; 145:341–355.30. Sinusas AJ, Bengel F, Nahrendorf M, et al. Multimodality cardiovascular molecular imaging, part I. Circ Cardiovasc Imaging. 2008; 1:244–256.31. Nahrendorf M, Sosnovik DE, French BA, et al. Multimodality cardiovascular molecular imaging, part II. Circ Cardiovasc Imaging. 2009; 2:56–70.32. Jaffer FA, Nahrendorf M, Sosnovik D, Kelly KA, Aikawa E, Weissleder R. Cellular imaging of inflammation in atherosclerosis using magnetofluorescent nanomaterials. Mol Imaging. 2006; 5:85–92.33. Hwang BH, Kim MH, Chang K. Molecular imaging of high-risk atherosclerotic plaques: is it clinically translatable? Korean Circ J. 2011; 41:497–502.34. Hlatky MA, Douglas PS, Cook NL, et al. Future directions for cardiovascular disease comparative effectiveness research: report of a workshop sponsored by the National Heart, Lung, and Blood Institute. J Am Coll Cardiol. 2012; 60:569–580.35. Kubo T, Maehara A, Mintz GS, et al. The dynamic nature of coronary artery lesion morphology assessed by serial virtual histology intravascular ultrasound tissue characterization. J Am Coll Cardiol. 2010; 55:1590–1597.36. Perlini S, Meyer TE, Foëx P. Effects of preload, afterload and inotropy on dynamics of ischemic segmental wall motion. J Am Coll Cardiol. 1997; 29:846–855.37. Chatzizisis YS, Baker AB, Sukhova GK, et al. Augmented expression and activity of extracellular matrix-degrading enzymes in regions of low endothelial shear stress colocalize with coronary atheromata with thin fibrous caps in pigs. Circulation. 2011; 123:621–630.38. Chatzizisis YS, Jonas M, Coskun AU, et al. Prediction of the localization of high-risk coronary atherosclerotic plaques on the basis of low endothelial shear stress: an intravascular ultrasound and histopathology natural history study. Circulation. 2008; 117:993–1002.39. Koskinas KC, Sukhova GK, Baker AB, et al. Thin-capped atheromata with reduced collagen content in pigs develop in coronary arterial regions exposed to persistently low endothelial shear stress. Arterioscler Thromb Vasc Biol. 2013; 33:1494–1504.40. Stone PH, Saito S, Takahashi S, et al. Prediction of progression of coronary artery disease and clinical outcomes using vascular profiling of endothelial shear stress and arterial plaque characteristics: the PREDICTION Study. Circulation. 2012; 126:172–181.41. Vergallo R, Papafaklis MI, Yonetsu T, et al. Endothelial shear stress and coronary plaque characteristics in humans: combined frequency-domain optical coherence tomography and computational fluid dynamics study. Circ Cardiovasc Imaging. 2014; 7:905–911.42. Phinikaridou A, Hua N, Pham T, Hamilton JA. Regions of low endothelial shear stress colocalize with positive vascular remodeling and atherosclerotic plaque disruption: an in vivo magnetic resonance imaging study. Circ Cardiovasc Imaging. 2013; 6:302–310.43. Koskinas KC, Chatzizisis YS, Baker AB, Edelman ER, Stone PH, Feldman CL. The role of low endothelial shear stress in the conversion of atherosclerotic lesions from stable to unstable plaque. Curr Opin Cardiol. 2009; 24:580–590.44. Kwak BR, Bäck M, Bochaton-Piallat ML, et al. Biomechanical factors in atherosclerosis: mechanisms and clinical implications. Eur Heart J. 2014; 35:3013–3020.45. Kumar A, Thompson EW, Lefieux A, et al. High coronary shear stress in patients with coronary artery disease predicts myocardial infarction. J Am Coll Cardiol. 2018; 72:1926–1935.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current Status and Future Directions of Primary Care
- Past, Present and Future of Intravascular Ultrasound and Optical Coherence Tomography
- Current status and future directions of ovarian cancer prognostic models
- Ultrasonography: current status, challenges, and future directions
- Aortic Stenosis and Transcatheter Aortic Valve Implantation: Current Status and Future Directions in Korea