J Korean Acad Prosthodont.
2019 Oct;57(4):328-334. 10.4047/jkap.2019.57.4.328.
The effects of saline soaking on the removal torque of titanium implants in rabbit tibia after 10 days
- Affiliations
-
- 1Department of Prosthodontics, School of Dentistry, Kyungpook National University, Daegu, Republic of Korea. sungamcho@naver.com
- KMID: 2461138
- DOI: http://doi.org/10.4047/jkap.2019.57.4.328
Abstract
- PURPOSE
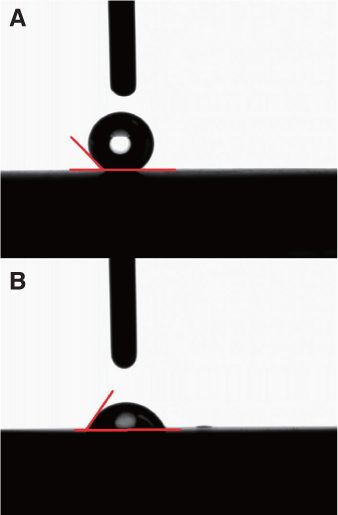
The aim of this study was to confirm if Laser-treated implants were soaked in 0.9% NaCl solution for 2 weeks could increase the surface hydrophilicity, and the Remoal Torque of each implant that inserted in rabbit tibia for initial healing period of 10 days.
MATERIALS AND METHODS
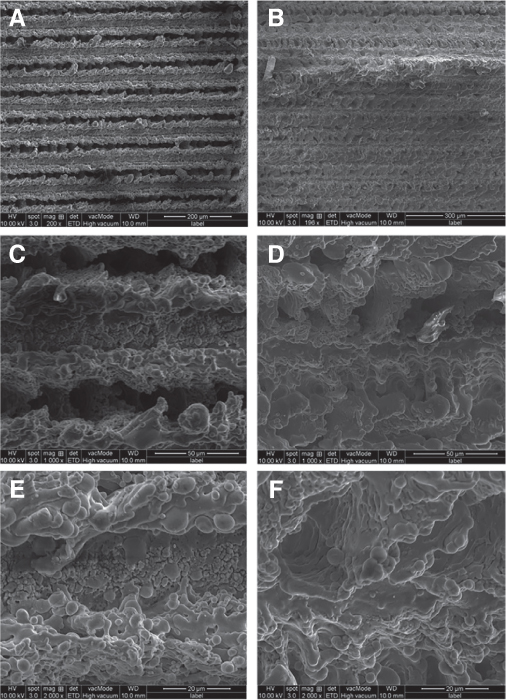
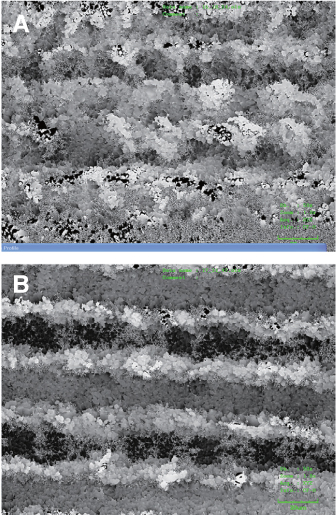
Twenty machined titanium surface screws were produced with a diameter 3 mm, length 8 mm. Ten screws had their surface treated with a laser only (laser treated group), and the other 10 were soaked in saline for 2 weeks after surface treatment with a laser (laser treated + saline soaked group). Implants were inserted in rabbit tibia (ten adult New Zealand white rabbits), and the RTQ of each implant was measured after 10 days. The wettability among implants was compared by measuring the contact angle. Surface composition and surface topography were analyzed.
RESULTS
After 10 days, the laser treat + soaking group implants had a significantly higher mean RTQ than the laser treated implants (P = .002, < .05). There were no significant morphological differences between groups, and no remarkable differences were found between the two groups in the SEM analysis.
CONCLUSION
Saline soaking implants is expected to produce excellent RTQ and surface analysis results.
Keyword
MeSH Terms
Figure
Reference
-
1. Parithimarkalaignan S, Padmanabhan TV. Osseointegration: An update. J Indian Prosthodont Soc. 2013; 13:2–6.
Article2. Cooper LF. A role for surface topography in creating and maintaining bone at titanium endosseous implants. J Prosthet Dent. 2000; 84:522–534.
Article3. Wennerberg A, Jimbo R, Stübinger S, Obrecht M, Dard M, Berner S. Nanostructures and hydrophilicity influence osseointegration: a biomechanical study in the rabbit tibia. Clin Oral Implants Res. 2014; 25:1041–1050.
Article4. Anil S, Anand PS, Alghamdi H, Jansen JA. Dental implant surface enhancement and osseointegration. In Tech. 2011. p. 83–108. http://www.intechopen.com/books/implant-dentistry-a-rapidly-evolving-practice/dental-implant-surface-enhancement-and-osseointegration.5. Cho SA, Jung SK. A removal torque of the laser-treated titanium implants in rabbit tibia. Biomaterials. 2003; 24:4859–4863.
Article6. Berglundh T, Abrahamsson I, Lang NP, Lindhe J. De novo alveolar bone formation adjacent to endosseous implants. Clin Oral Implants Res. 2003; 14:251–262.
Article7. Wennerberg A, Albrektsson T. Effects of titanium surface topography on bone integration: a systematic review. Clin Oral Implants Res. 2009; 20:172–184.
Article8. Sittig C, Textor M, Spencer ND, Wieland M, Vallotton PH. Surface characterization of implant materials c.p. Ti, Ti-6Al-7Nb and Ti-6Al-4V with different pretreatments. J Mater Sci Mater Med. 1999; 10:35–46.9. Massaro C, Rotolo P, De Riccardis F, Milella E, Napoli A, Wieland M, Textor M, Spencer ND, Brunette DM. Comparative investigation of the surface properties of commercial titanium dental implants. Part I: chemical composition. J Mater Sci Mater Med. 2002; 13:535–548.10. Le Guéhennec L, Soueidan A, Layrolle P, Amouriq Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent Mater. 2007; 23:844–854.
Article11. Rupp F, Scheideler L, Eichler M, Geis-Gerstorfer J. Wetting behavior of dental implants. Int J Oral Maxillofac Implants. 2011; 26:1256–1266.12. Zhao G, Schwartz Z, Wieland M, Rupp F, Geis-Gerstorfer J, Cochran DL, Boyan BD. High surface energy enhances cell response to titanium substrate microstructure. J Biomed Mater Res A. 2005; 74:49–58.
Article13. Sartoretto SC, Alves AT, Resende RF, Calasans-Maia J, Granjeiro JM, Calasans-Maia MD. Early osseointegration driven by the surface chemistry and wettability of dental implants. J Appl Oral Sci. 2015; 23:279–287.
Article14. Elias CN. Factors affecting the success of dental implants. In : Turkyilmaz I, editor. Implant dentistry: a rapidly evolving practice. Rijeka: InTech;2011. p. 319–364.15. Weber HP, Morton D, Gallucci GO, Roccuzzo M, Cordaro L, Grutter L. Consensus statements and recommended clinical procedures regarding loading protocols. Int J Oral Maxillofac Implants. 2009; 24:180–183.16. Kwon JU, Cho SA. Comparison of removal torque of saline-soaking RBM implants and RBM implants in rabbit tibias. J Korean Acad Prosthodont. 2018; 56:1–7.
Article17. Sela MN, Badihi L, Rosen G, Steinberg D, Kohavi D. Adsorption of human plasma proteins to modified titanium surfaces. Clin Oral Implants Res. 2007; 18:630–638.
Article18. Buser D, Broggini N, Wieland M, Schenk RK, Denzer AJ, Cochran DL, Hoffmann B, Lussi A, Steinemann SG. Enhanced bone apposition to a chemically modified SLA titanium surface. J Dent Res. 2004; 83:529–533.
Article19. Donos N, Hamlet S, Lang NP, Salvi GE, Huynh-Ba G, Bosshardt DD, Ivanovski S. Gene expression profile of osseointegration of a hydrophilic compared with a hydrophobic microrough implant surface. Clin Oral Implants Res. 2011; 22:365–372.
Article20. Ivanoff CJ, Sennerby L, Lekholm U. Influence of mono- and bicortical anchorage on the integration of titanium implants. A study in the rabbit tibia. Int J Oral Maxillofac Surg. 1996; 25:229–235.
Article21. Albrektsson T, Brånemark PI, Hansson HA, Lindström J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand. 1981; 52:155–170.
Article22. Textor M, Sittig C, Frauchiger V, Tosatti S, Brunette DM. Properties and biological significance of natural oxide films on titanium and its alloys. In : Brunette DM, Tengvall P, Textor M, Thomsen P, editors. Titanium in medicine: material science, surface science, engineering, biological responses and medical applications. New York: Springer;2001. p. 171–230.23. Lang NP, Salvi GE, Huynh-Ba G, Ivanovski S, Donos N, Bosshardt DD. Early osseointegration to hydrophilic and hydrophobic implant surfaces in humans. Clin Oral Implants Res. 2011; 22:349–356.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of removal torque of saline-soaking RBM implants and RBM implants in rabbit tibias
- On the effect of saline immersion to the removal torque for resorbable blasting media and acid treated implants
- Comparison of removal torques of SLActive® implant and blasted, laser-treated titanium implant in rabbit tibia bone healed with concentrated growth factor application
- Comparison of histologic observation and insertional and removal torque values between titanium grade 2 and 4 microimplants
- The influence of intentional mobilization of implant fixtures before osseointegration