Investig Clin Urol.
2019 Nov;60(6):472-479. 10.4111/icu.2019.60.6.472.
Effects of short-term atorvastatin use in patients with calcium stones: A randomized placebo-controlled clinical trial
- Affiliations
-
- 1Urology and Nephrology Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran. s.tavasoli@sbmu.ac.ir
- 2Modern Epidemiology Research Centre, Aja University of Medical Science, Tehran, Iran.
- 3Urology and Nephrology Research Center, Shahid Labbafinejad Medical Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
- KMID: 2461059
- DOI: http://doi.org/10.4111/icu.2019.60.6.472
Abstract
- PURPOSE
A few experimental and observational studies have reported that atorvastatin prevents calcium oxalate stone formation. Our study is the first to investigate the effect of atorvastatin on 24-hour urinary metabolites, urinary malondialdehyde (U-MDA) (an oxidative stress marker) and urinary neutrophil gelatinase-associated lipocalin (U-NGAL) (a renal tubular injury marker) in patients with calcium stones and hyperoxaluria.
MATERIALS AND METHODS
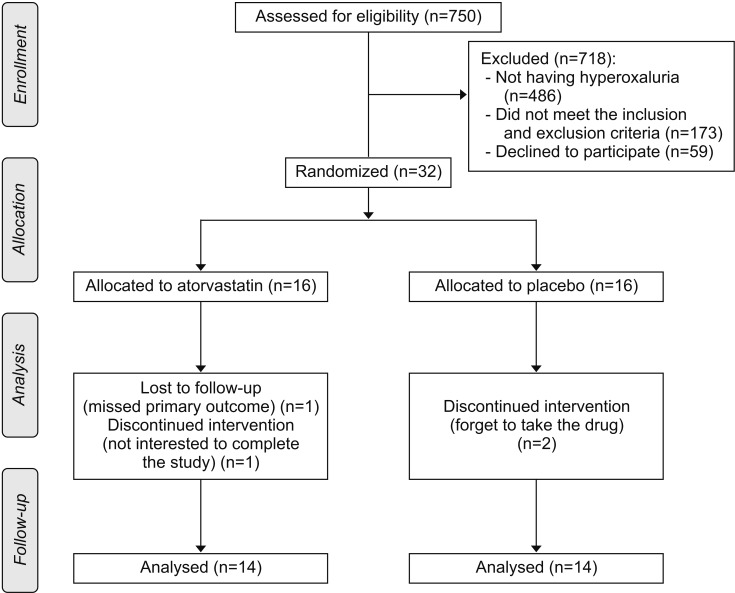
This randomized, double-blind, placebo-controlled, parallel-group clinical trial included 32 adults with recurrent calcium stone formation and hyperoxaluria. All participants received a 3-month course of either atorvastatin (20 mg/d) or placebo of an identical shape. Both groups received the usual nutritional care based on the European Association of Urology guidelines.
RESULTS
Twenty-eight participants completed the study. Serum levels of total and low-density lipoprotein cholesterol decreased in the atorvastatin group, and these changes were significantly different between groups (p<0.001). No statistically significant differences were observed between intergroup changes of the 24-hour urinary metabolite analysis, the U-MDA to creatinine ratio and the U-NGAL to creatinine ratio.
CONCLUSIONS
Atorvastatin administration at a dose of 20 mg/d for 3 months did not affect 24-hour urinary metabolite, U-MDA and U-NGAL levels in recurrent calcium stone formers. However, this study could not disprove the preventive role of atorvastatin in kidney stone formation. Future studies should consider a larger sample size, longer follow-up, different drug doses, and measurements of multiple biomarkers of oxidative stress and tubular injury.
Keyword
MeSH Terms
-
Adult
Atorvastatin Calcium*
Biomarkers
Calcium Oxalate
Calcium*
Cholesterol
Creatinine
Follow-Up Studies
Humans
Hydroxymethylglutaryl-CoA Reductase Inhibitors
Hyperoxaluria
Kidney Calculi
Lipocalins
Lipoproteins
Malondialdehyde
Neutrophils
Oxidative Stress
Sample Size
Urolithiasis
Urology
Atorvastatin Calcium
Biomarkers
Calcium
Calcium Oxalate
Cholesterol
Creatinine
Hydroxymethylglutaryl-CoA Reductase Inhibitors
Lipocalins
Lipoproteins
Malondialdehyde
Figure
Reference
-
1. Pourmand G, Pourmand B. Epidemiology of stone disease in Iran. In : Talati JJ, Tiselius HG, Albala DM, Ye Z, editors. Urolithiasis: basic science and clinical practice. London: Springer;2012. p. 85–87.2. Masterson JH, Woo JR, Chang DC, Chi T, L'Esperance JO, Stoller ML, et al. Dyslipidemia is associated with an increased risk of nephrolithiasis. Urolithiasis. 2015; 43:49–53. PMID: 25193087.
Article3. Sur RL, Masterson JH, Palazzi KL, L'Esperance JO, Auge BK, Chang DC, et al. Impact of statins on nephrolithiasis in hyperlipidemic patients: a 10-year review of an equal access health care system. Clin Nephrol. 2013; 79:351–355. PMID: 23195830.
Article4. Cohen AJ, Adamsky MA, Nottingham CU, Pruitt J, Lapin B, Wang CH, et al. Impact of statin intake on kidney stone formation. Urology. 2019; 124:57–61. PMID: 29421299.
Article5. Tsujihata M, Momohara C, Yoshioka I, Tsujimura A, Nonomura N, Okuyama A. Atorvastatin inhibits renal crystal retention in a rat stone forming model. J Urol. 2008; 180:2212–2217. PMID: 18804815.
Article6. Tsujihata M, Yoshioka I, Tsujimura A, Nonomura N, Okuyama A. Why does atorvastatin inhibit renal crystal retention. Urol Res. 2011; 39:379–383. PMID: 21400107.
Article7. Temiz MZ, Yuruk E, Ertas K, Zengi O, Semercioz A. Effects of statin treatment with atorvastatin on urolithiasis-associated urinary metabolic risk factors: an experimental study. Int Urol Nephrol. 2018; 50:231–236. PMID: 29197934.
Article8. Kumar A, Hecht C, Priyamvada S, Anbazhagan AN, Alakkam A, Borthakur A, et al. Probiotic Bifidobacterium species stimulate human SLC26A3 gene function and expression in intestinal epithelial cells. Am J Physiol Cell Physiol. 2014; 307:C1084–C1092. PMID: 25143346.
Article9. Chow SC, Shao J, Wang H. Sample size calculations in clinical research. 2nd ed. Boca Raton: Taylor & Francis;2007. p. 60.10. Skolarikos A, Straub M, Knoll T, Sarica K, Seitz C, Petřík A, et al. Metabolic evaluation and recurrence prevention for urinary stone patients: EAU guidelines. Eur Urol. 2015; 67:750–763. PMID: 25454613.
Article11. Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin Ther. 1999; 21:1074–1090. PMID: 10440628.
Article12. Noori N, Honarkar E, Goldfarb DS, Kalantar-Zadeh K, Taheri M, Shakhssalim N, et al. Urinary lithogenic risk profile in recurrent stone formers with hyperoxaluria: a randomized controlled trial comparing DASH (Dietary Approaches to Stop Hypertension)-style and low-oxalate diets. Am J Kidney Dis. 2014; 63:456–463. PMID: 24560157.
Article13. Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta. 1978; 90:37–43. PMID: 719890.14. Tavasoli S, Basiri A, Khoshdel A, Taheri M. Evaluating the association of body mass index, waist circumference and waist to stature ratio with urine composition in patients with urolithiasis. Iran J Kidney Dis. 2017; 11:371–378. PMID: 29038393.15. Santhosh Kumar M, Selvam R. Supplementation of vitamin E and selenium prevents hyperoxaluria in experimental urolithic rats. J Nutr Biochem. 2003; 14:306–313. PMID: 12873711.
Article16. Fry DW, Richardson KE. Isolation and characterization of glycolic acid oxidase from human liver. Biochim Biophys Acta. 1979; 568:135–144. PMID: 444540.
Article17. Steinhubl SR. Why have antioxidants failed in clinical trials? Am J Cardiol. 2008; 101(10A):14D–19D.
Article18. Parthasarathy S, Khan-Merchant N, Penumetcha M, Khan BV, Santanam N. Did the antioxidant trials fail to validate the oxidation hypothesis. Curr Atheroscler Rep. 2001; 3:392–398. PMID: 11487450.
Article19. Kok DJ, Boellaard W, Ridwan Y, Levchenko VA. Timelines of the “free-particle” and “fixed-particle” models of stone-formation: theoretical and experimental investigations. Urolithiasis. 2017; 45:33–41. PMID: 27915394.
Article20. Ma MC, Chen YS, Huang HS. Erythrocyte oxidative stress in patients with calcium oxalate stones correlates with stone size and renal tubular damage. Urology. 2014; 83:510.e9–510.e17.
Article21. Boonla C, Wunsuwan R, Tungsanga K, Tosukhowong P. Urinary 8-hydroxydeoxyguanosine is elevated in patients with nephrolithiasis. Urol Res. 2007; 35:185–191. PMID: 17541572.
Article22. Carnevale R, Pignatelli P, Di Santo S, Bartimoccia S, Sanguigni V, Napoleone L, et al. Atorvastatin inhibits oxidative stress via adiponectin-mediated NADPH oxidase down-regulation in hypercholesterolemic patients. Atherosclerosis. 2010; 213:225–234. PMID: 20832062.
Article23. Zhu W, Liu M, Wang GC, Che JP, Xu YF, Peng B, et al. Urinary neutrophil gelatinase-associated lipocalin, a biomarker for systemic inflammatory response syndrome in patients with nephrolithiasis. J Surg Res. 2014; 187:237–243. PMID: 24239146.
Article24. Kandur Y, Gonen S, Fidan K, Soylemezoglu O. Evaluation of urinary KIM-1, NGAL, and IL-18 levels in determining early renal injury in pediatric cases with hypercalciuria and/or renal calculi. Clin Nephrol. 2016; 86:62–69. PMID: 27345186.
Article25. Carrasco-Valiente J, Anglada-Curado FJ, Aguilar-Melero P, González-Ojeda R, Muntané-Relat J, Padillo-Ruiz FJ, et al. [State of acute phase markers and oxidative stress in patients with kidney stones in the urinary tract]. Actas Urol Esp. 2012; 36:296–301. Spanish. PMID: 22301017.
Article26. Tungsanga K, Sriboonlue P, Futrakul P, Yachantha C, Tosukhowong P. Renal tubular cell damage and oxidative stress in renal stone patients and the effect of potassium citrate treatment. Urol Res. 2005; 33:65–69. PMID: 15565439.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of Atorvastatin for the Treatment of Patients With Asthma: A Double-Blind Randomized Clinical Trial
- Effects of cetirizine in dogs with chronic atopic dermatitis: a randomized, double blind, placebo-controlled trial
- Effects of Atorvastatin in Patients with Acute Spinal Cord Injury
- The Efficacy of Terazosin in the Treatment of Benign Prostatic Hyperplasia: A Randomized, Placebo-controlled Double Blind Study
- Efficacy of Roflumilast in Bronchiectasis Patients with Frequent Exacerbations: A Double-Blinded, Randomized, Placebo-Controlled Pilot Clinical Trial