Diabetes Metab J.
2019 Oct;43(5):568-577. 10.4093/dmj.2019.0143.
Mitochondrial Toxins and Healthy Lifestyle Meet at the Crossroad of Hormesis
- Affiliations
-
- 1Department of Preventive Medicine, School of Medicine, Kyungpook National University, Daegu, Korea. lee_dh@knu.ac.kr
- 2BK21 Plus KNU Biomedical Convergence Program, Department of Biomedical Science, Kyungpook National University, Daegu, Korea.
- KMID: 2460950
- DOI: http://doi.org/10.4093/dmj.2019.0143
Abstract
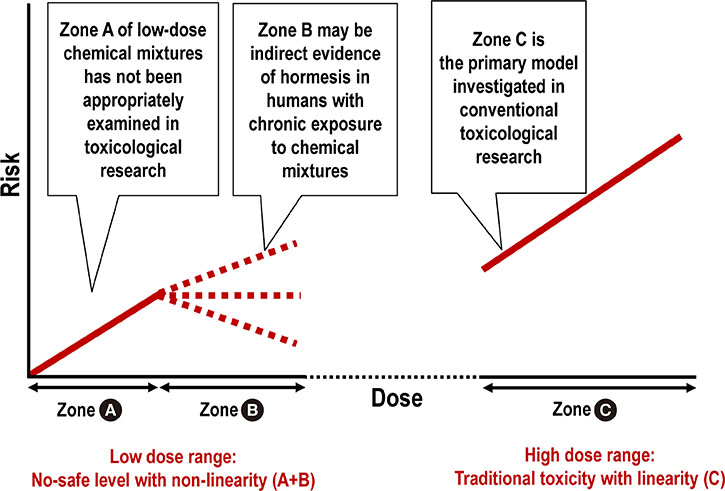
- Mitochondrial function is crucial for the maintenance of cellular homeostasis under physiological and stress conditions. Thus, chronic exposure to environmental chemicals that affect mitochondrial function can have harmful effects on humans. We argue that the concept of hormesis should be revisited to explain the non-linear responses to mitochondrial toxins at a low-dose range and develop practical methods to protect humans from the negative effects of mitochondrial toxins. Of the most concern to humans are lipophilic chemical mixtures and heavy metals, owing to their physical properties. Even though these chemicals tend to demonstrate no safe level in humans, a non-linear dose-response has been also observed. Stress response activation, i.e., hormesis, can explain this non-linearity. Recently, hormesis has reemerged as a unifying concept because diverse stressors can induce similar stress responses. Besides potentially harmful environmental chemicals, healthy lifestyle interventions such as exercise, calorie restriction (especially glucose), cognitive stimulation, and phytochemical intake also activate stress responses. This conceptual link can lead to the development of practical methods that counterbalance the harm of mitochondrial toxins. Unlike chemical hormesis with its safety issues, the activation of stress responses via lifestyle modification can be safely used to combat the negative effects of mitochondrial toxins.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Can Environmental Pollutants Be a Factor Linking Obesity and COVID-19?
Duk-Hee Lee
J Korean Med Sci. 2021;36(43):e305. doi: 10.3346/jkms.2021.36.e305.Effect of Low-Dose Persistent Organic Pollutants on Mitochondrial Function: Human and
in Vitro Evidence
Se-A Kim, Hoyul Lee, Sung-Mi Park, Mi-Jin Kim, Yu-Mi Lee, Young-Ran Yoon, Hyun-Kyung Lee, Hyo-Bang Moon, In-Kyu Lee, Duk-Hee Lee
Diabetes Metab J. 2022;46(4):592-604. doi: 10.4093/dmj.2021.0132.
Reference
-
1. Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009; 30:293–342.2. Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT. EDC-2: the endocrine society's second scientific statement on endocrine-disrupting chemicals. Endocr Rev. 2015; 36:E1–E150.3. Heindel JJ, Blumberg B, Cave M, Machtinger R, Mantovani A, Mendez MA, Nadal A, Palanza P, Panzica G, Sargis R, Vandenberg LN, Vom Saal F. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol. 2017; 68:3–33.4. Kim JT, Lee HK. Childhood obesity and endocrine disrupting chemicals. Ann Pediatr Endocrinol Metab. 2017; 22:219–225.5. Lee DH, Jacobs DR Jr. Firm human evidence on harms of endocrine-disrupting chemicals was unlikely to be obtainable for methodological reasons. J Clin Epidemiol. 2019; 107:107–115.6. Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012; 148:1145–1159.7. Calabrese EJ. Hormesis is central to toxicology, pharmacology and risk assessment. Hum Exp Toxicol. 2010; 29:249–261.8. Meyer JN, Chan SSL. Sources, mechanisms, and consequences of chemical-induced mitochondrial toxicity. Toxicology. 2017; 391:2–4.9. Friedman JR, Nunnari J. Mitochondrial form and function. Nature. 2014; 505:335–343.10. Chandel NS. Evolution of mitochondria as signaling organelles. Cell Metab. 2015; 22:204–206.11. Yun J, Finkel T. Mitohormesis. Cell Metab. 2014; 19:757–766.12. Ristow M. Unraveling the truth about antioxidants: mitohormesis explains ROS-induced health benefits. Nat Med. 2014; 20:709–711.13. Shadel GS, Horvath TL. Mitochondrial ROS signaling in organismal homeostasis. Cell. 2015; 163:560–569.14. Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009; 417:1–13.15. Cortassa S, Aon MA, Winslow RL, O'Rourke B. A mitochondrial oscillator dependent on reactive oxygen species. Biophys J. 2004; 87:2060–2073.16. Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956; 11:298–300.17. Ristow M, Schmeisser K. Mitohormesis: promoting health and lifespan by increased levels of reactive oxygen species (ROS). Dose Response. 2014; 12:288–341.18. Wallace KB. Multiple targets for drug-induced mitochondrial toxicity. Curr Med Chem. 2015; 22:2488–2492.19. Meyer JN, Leung MC, Rooney JP, Sendoel A, Hengartner MO, Kisby GE, Bess AS. Mitochondria as a target of environmental toxicants. Toxicol Sci. 2013; 134:1–17.20. Brunst KJ, Baccarelli AA, Wright RJ. Integrating mitochondriomics in children's environmental health. J Appl Toxicol. 2015; 35:976–991.21. Attene-Ramos MS, Huang R, Michael S, Witt KL, Richard A, Tice RR, Simeonov A, Austin CP, Xia M. Profiling of the Tox21 chemical collection for mitochondrial function to identify compounds that acutely decrease mitochondrial membrane potential. Environ Health Perspect. 2015; 123:49–56.22. Meyer JN, Leuthner TC, Luz AL. Mitochondrial fusion, fission, and mitochondrial toxicity. Toxicology. 2017; 391:42–53.23. Zielonka J, Joseph J, Sikora A, Hardy M, Ouari O, Vasquez-Vivar J, Cheng G, Lopez M, Kalyanaraman B. Mitochondria-targeted triphenylphosphonium-based compounds: syntheses, mechanisms of action, and therapeutic and diagnostic applications. Chem Rev. 2017; 117:10043–10120.24. Fetterman JL, Sammy MJ, Ballinger SW. Mitochondrial toxicity of tobacco smoke and air pollution. Toxicology. 2017; 391:18–33.25. Rudel RA, Perovich LJ. Endocrine disrupting chemicals in indoor and outdoor air. Atmos Environ (1994). 2009; 43:170–181.26. Zhang Y, Ji X, Ku T, Li G, Sang N. Heavy metals bound to fine particulate matter from northern China induce season-dependent health risks: a study based on myocardial toxicity. Environ Pollut. 2016; 216:380–390.27. Ketterer B, Coles B, Meyer DJ. The role of glutathione in detoxication. Environ Health Perspect. 1983; 49:59–69.28. Macdonald TL. Chemical mechanisms of halocarbon metabolism. Crit Rev Toxicol. 1983; 11:85–120.29. Slezak BP, Hatch GE, DeVito MJ, Diliberto JJ, Slade R, Crissman K, Hassoun E, Birnbaum LS. Oxidative stress in female B6C3F1 mice following acute and subchronic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Toxicol Sci. 2000; 54:390–398.30. Santra A, Maiti A, Chowdhury A, Mazumder DN. Oxidative stress in liver of mice exposed to arsenic-contaminated water. Indian J Gastroenterol. 2000; 19:112–115.31. Mari M, Morales A, Colell A, Garcia-Ruiz C, Fernandez-Checa JC. Mitochondrial glutathione, a key survival antioxidant. Antioxid Redox Signal. 2009; 11:2685–2700.32. Sthijns MM, Weseler AR, Bast A, Haenen GR. Time in redox adaptation processes: from evolution to hormesis. Int J Mol Sci. 2016; 17:E1649.33. Lanphear BP. Low-level toxicity of chemicals: no acceptable levels? PLoS Biol. 2017; 15:e2003066.34. Canfield RL, Henderson CR Jr, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. N Engl J Med. 2003; 348:1517–1526.35. Lanphear BP, Rauch S, Auinger P, Allen RW, Hornung RW. Low-level lead exposure and mortality in US adults: a population-based cohort study. Lancet Public Health. 2018; 3:e177–e184.36. Pope CA 3rd, Burnett RT, Krewski D, Jerrett M, Shi Y, Calle EE, Thun MJ. Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke: shape of the exposure-response relationship. Circulation. 2009; 120:941–948.37. Vlaanderen J, Portengen L, Rothman N, Lan Q, Kromhout H, Vermeulen R. Flexible meta-regression to assess the shape of the benzene-leukemia exposure-response curve. Environ Health Perspect. 2010; 118:526–532.38. Lee DH, Porta M, Jacobs DR Jr, Vandenberg LN. Chlorinated persistent organic pollutants, obesity, and type 2 diabetes. Endocr Rev. 2014; 35:557–601.39. Bowers TS, Beck BD. What is the meaning of non-linear dose-response relationships between blood lead concentrations and IQ? Neurotoxicology. 2006; 27:520–524.40. Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr, Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev. 2012; 33:378–455.41. Calabrese EJ, Mattson MP. How does hormesis impact biology, toxicology, and medicine? NPJ Aging Mech Dis. 2017; 3:13.42. Kim SA, Lee YM, Choi JY, Jacobs DR Jr, Lee DH. Evolutionarily adapted hormesis-inducing stressors can be a practical solution to mitigate harmful effects of chronic exposure to low dose chemical mixtures. Environ Pollut. 2018; 233:725–734.43. Calabrese EJ. Paradigm lost, paradigm found: the re-emergence of hormesis as a fundamental dose response model in the toxicological sciences. Environ Pollut. 2005; 138:379–411.44. Needleman HL. The removal of lead from gasoline: historical and personal reflections. Environ Res. 2000; 84:20–35.45. Lee YM, Kim KS, Jacobs DR Jr, Lee DH. Persistent organic pollutants in adipose tissue should be considered in obesity research. Obes Rev. 2017; 18:129–139.46. Lee DH, Jacobs DR Jr. New approaches to cope with possible harms of low-dose environmental chemicals. J Epidemiol Community Health. 2019; 73:193–197.47. Gems D, Partridge L. Stress-response hormesis and aging: “that which does not kill us makes us stronger”. Cell Metab. 2008; 7:200–203.48. Martins I, Galluzzi L, Kroemer G. Hormesis, cell death and aging. Aging (Albany NY). 2011; 3:821–828.49. Howitz KT, Sinclair DA. Xenohormesis: sensing the chemical cues of other species. Cell. 2008; 133:387–391.50. Tapia PC. Sublethal mitochondrial stress with an attendant stoichiometric augmentation of reactive oxygen species may precipitate many of the beneficial alterations in cellular physiology produced by caloric restriction, intermittent fasting, exercise and dietary phytonutrients: “mitohormesis” for health and vitality. Med Hypotheses. 2006; 66:832–843.51. Lopez-Lluch G, Navas P. Calorie restriction as an intervention in ageing. J Physiol. 2016; 594:2043–2060.52. Merry TL, Ristow M. Mitohormesis in exercise training. Free Radic Biol Med. 2016; 98:123–130.53. Calabrese V, Cornelius C, Dinkova-Kostova AT, Iavicoli I, Di Paola R, Koverech A, Cuzzocrea S, Rizzarelli E, Calabrese EJ. Cellular stress responses, hormetic phytochemicals and vitagenes in aging and longevity. Biochim Biophys Acta. 2012; 1822:753–783.54. Hartman JH, Smith LL, Gordon KL, Laranjeiro R, Driscoll M, Sherwood DR, Meyer JN. Swimming exercise and transient food deprivation in Caenorhabditis elegans promote mitochondrial maintenance and protect against chemical-induced Mitotoxicity. Sci Rep. 2018; 8:8359.55. Chen L, Mo H, Zhao L, Gao W, Wang S, Cromie MM, Lu C, Wang JS, Shen CL. Therapeutic properties of green tea against environmental insults. J Nutr Biochem. 2017; 40:1–13.56. Hoffman JB, Hennig B. Protective influence of healthful nutrition on mechanisms of environmental pollutant toxicity and disease risks. Ann N Y Acad Sci. 2017; 1398:99–107.57. Taylor RW, Turnbull DM. Mitochondrial DNA mutations in human disease. Nat Rev Genet. 2005; 6:389–402.58. Picard M, McManus MJ, Gray JD, Nasca C, Moffat C, Kopinski PK, Seifert EL, McEwen BS, Wallace DC. Mitochondrial functions modulate neuroendocrine, metabolic, inflammatory, and transcriptional responses to acute psychological stress. Proc Natl Acad Sci U S A. 2015; 112:E6614–E6623.59. Hood DA, Memme JM, Oliveira AN, Triolo M. Maintenance of skeletal muscle mitochondria in health, exercise, and aging. Annu Rev Physiol. 2019; 81:19–41.60. Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005; 309:481–484.61. Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly-Y M, Gidlof S, Oldfors A, Wibom R, Tornell J, Jacobs HT, Larsson NG. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004; 429:417–423.62. Safdar A, Bourgeois JM, Ogborn DI, Little JP, Hettinga BP, Akhtar M, Thompson JE, Melov S, Mocellin NJ, Kujoth GC, Prolla TA, Tarnopolsky MA. Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice. Proc Natl Acad Sci U S A. 2011; 108:4135–4140.63. Kelly MP, Barker M. Why is changing health-related behaviour so difficult? Public Health. 2016; 136:109–116.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pre-pregnancy lifestyle of couple for a healthy pregnancy
- Treatment of Winkles and Hyperhidrosis with Botulinum Toxin Type A
- Beneficial Effects of Low-Grade Mitochondrial Stress on Metabolic Diseases and Aging
- Low-dose Radiation-induced Hormetic Effect in the Rat Ovarian Follicle
- Healthy lifestyles in childhood cancer survivors in South Korea: a comparison between reports from children and their parents