Cancer Res Treat.
2019 Oct;51(4):1479-1487. 10.4143/crt.2018.649.
Prognostic Value of Baseline and Interim Total Metabolic Tumor Volume and Total Lesion Glycolysis Measured on ¹â¸F-FDG PET-CT in Patients with Follicular Lymphoma
- Affiliations
-
- 1Department of Hematology, The First Affiliated Hospital of Nanjing Medical University, Jiangsu Province Hospital, Collaborative Innovation Center for Cancer Personalized Medicine, Nanjing, China. xuwei10000@hotmail.com, lijianyonglm@126.com
- 2Department of Nuclear Medicine, The First Affiliated Hospital of Nanjing Medical University, Jiangsu Province Hospital, Nanjing, China.
- KMID: 2460596
- DOI: http://doi.org/10.4143/crt.2018.649
Abstract
- PURPOSE
The purpose of this study was to investigate the prognostic significance of total metabolic tumor volume (TMTV) and total lesion glycolysis (TLG) in patients with follicular lymphoma (FL) at baseline and mid-treatment with ¹â¸F-fluorodeoxyglucose positron emission tomography-computed tomography (PET-CT) scans.
MATERIALS AND METHODS
The study analyzed data from 48 patients with FL who were treated in Jiangsu Province Hospital and reviewed their baseline PET-CT scans. TMTV and TLG were computed by using the absolute value of 2.0, 2.5, and 3.0 thresholding method, respectively.
RESULTS
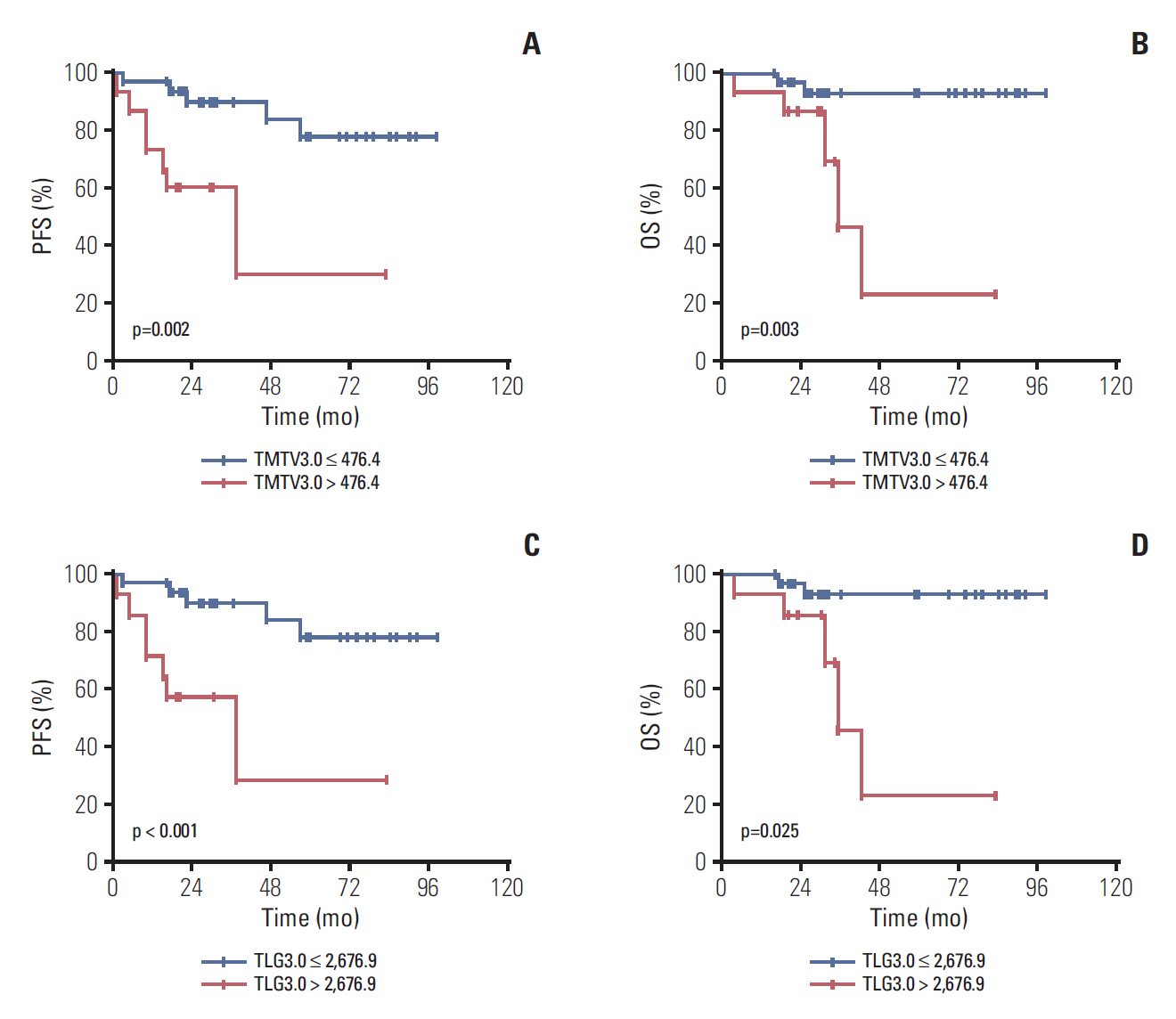
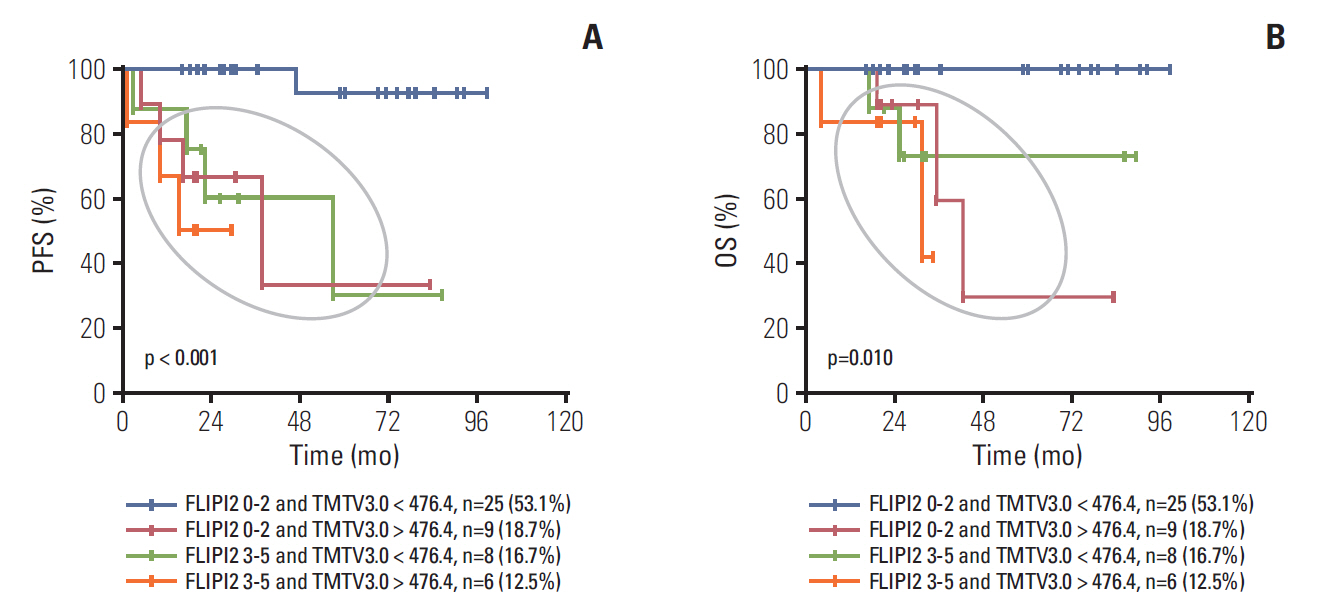
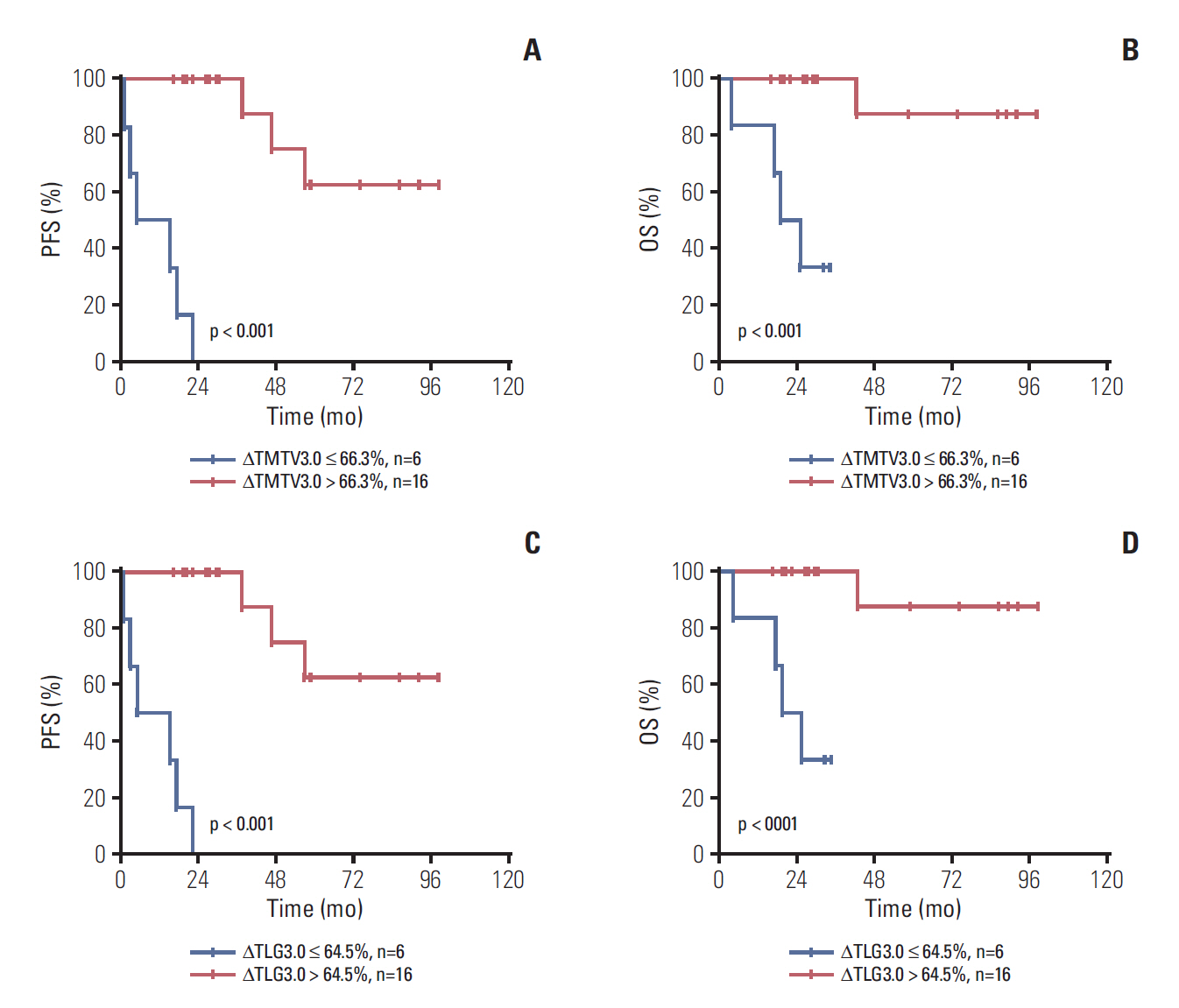
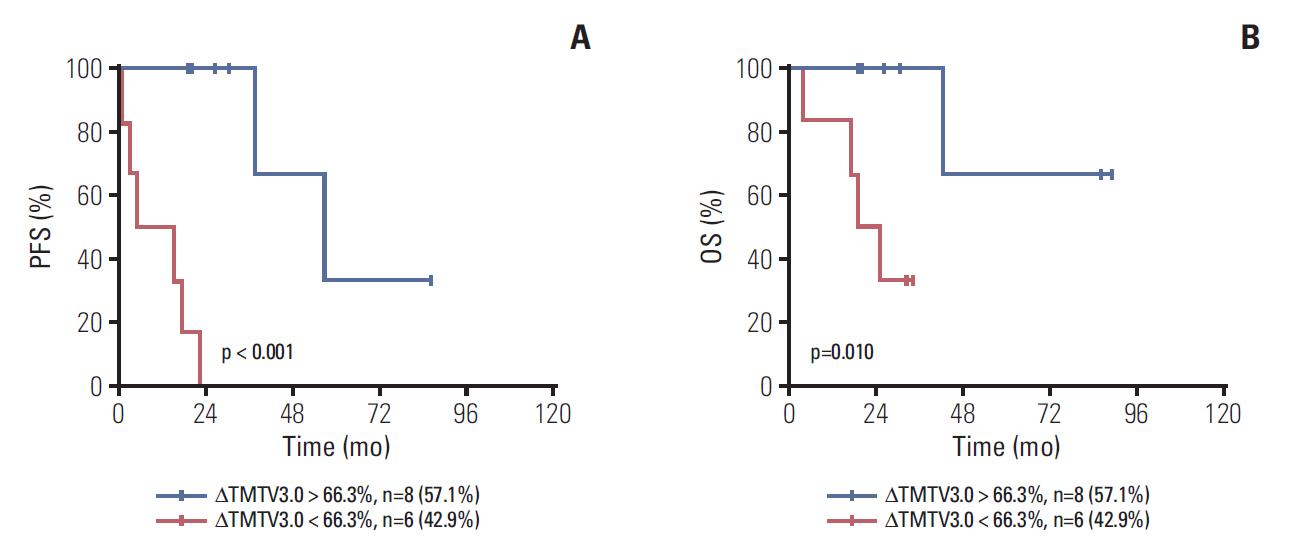
Median age was 53 years, 75.0% of patients had stage III to IV disease, 43.8% had a Follicular Lymphoma International Prognostic Index 1 (FLIPI1) score of 3 to 5 and 20.8% had a FLIPI2 score of 3 to 5. Receiver operating characteristic (ROC) curve analysis showed the optimal cut-off values for TMTV3.0 and TLG3.0 were 476.4 (sensitivity, 85.7%; specificity, 78.0%; area under the curve [AUC], 0.760; p=0.003) and 2,676.9 (sensitivity, 71.4%; specificity, 78.0%; AUC, 0.760; p=0.003). On multivariable analysis, TMTV3.0 and TLG3.0 were independent predictors of both progression-free survival (PFS) (hazard ratio [HR], 5.406; 95% confidence interval [CI], 1.326 to 22.040; p=0.019 and HR, 6.502; 95% CI, 1.079 to 39.182; p=0.042) and overall survival (OS) (HR, 4.111; 95% CI, 1.125 to 15.027; p=0.033 and HR, 5.885; 95% CI, 1.014 to 34.148; p=0.049). ROC curve analysis showed the optimal cut-off values for ΔTMTV3.0 and ΔTLG3.0 were 66.3% (sensitivity, 85.7%; specificity, 63.4%; AUC, 0.774; p < 0.001) and 64.5% (sensitivity, 85.7%; specificity, 65.9%; AUC, 0.777; p < 0.001).
CONCLUSION
Baseline TMTV and TLG are strong predictors of PFS and OS in FL. Furthermore, interim TMTV (ΔTMTV > 66.3%) and TLG (ΔTLG > 64.5%) reduction are valuable tools for early treatment response assessment in FL patients.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Smith A, Crouch S, Lax S, Li J, Painter D, Howell D, et al. Lymphoma incidence, survival and prevalence 2004-2014: sub-type analyses from the UK's Haematological Malignancy Research Network. Br J Cancer. 2015; 112:1575–84.
Article2. The World Health Organization classification of malignant lymphomas in japan: incidence of recently recognized entities. Lymphoma Study Group of Japanese Pathologists. Pathol Int. 2000; 50:696–702.3. Chen CY, Yao M, Tang JL, Tsay W, Wang CC, Chou WC, et al. Chromosomal abnormalities of 200 Chinese patients with non-Hodgkin's lymphoma in Taiwan: with special reference to T-cell lymphoma. Ann Oncol. 2004; 15:1091–6.
Article4. Au WY, Ma SY, Chim CS, Choy C, Loong F, Lie AK, et al. Clinicopathologic features and treatment outcome of mature T-cell and natural killer-cell lymphomas diagnosed according to the World Health Organization classification scheme: a single center experience of 10 years. Ann Oncol. 2005; 16:206–14.
Article5. Takeuchi M, Sato Y, Yoshino T. The frequency of malignant lymphoma subtypes based on World Health Organization (WHO) classification. Nihon Rinsho. 2014; 72:436–40.6. Federico M, Luminari S, Dondi A, Tucci A, Vitolo U, Rigacci L, et al. R-CVP versus R-CHOP versus R-FM for the initial treatment of patients with advanced-stage follicular lymphoma: results of the FOLL05 trial conducted by the Fondazione Italiana Linfomi. J Clin Oncol. 2013; 31:1506–13.
Article7. Casulo C, Day B, Dawson KL, Zhou X, Flowers CR, Farber CM, et al. Disease characteristics, treatment patterns, and outcomes of follicular lymphoma in patients 40 years of age and younger: an analysis from the National Lymphocare Study-dagger. Ann Oncol. 2015; 26:2311–7.8. Casulo C, Byrtek M, Dawson KL, Zhou X, Farber CM, Flowers CR, et al. Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: an analysis from the National LymphoCare Study. J Clin Oncol. 2015; 33:2516–22.
Article9. Solal-Celigny P, Roy P, Colombat P, White J, Armitage JO, Arranz-Saez R, et al. Follicular lymphoma international prognostic index. Blood. 2004; 104:1258–65.
Article10. Federico M, Bellei M, Marcheselli L, Luminari S, LopezGuillermo A, Vitolo U, et al. Follicular lymphoma international prognostic index 2: a new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. J Clin Oncol. 2009; 27:4555–62.
Article11. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014; 32:3059–68.
Article12. Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Mueller SP, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014; 32:3048–58.
Article13. Esfahani SA, Heidari P, Halpern EF, Hochberg EP, Palmer EL, Mahmood U. Baseline total lesion glycolysis measured with (18)F-FDG PET/CT as a predictor of progression-free survival in diffuse large B-cell lymphoma: a pilot study. Am J Nucl Med Mol Imaging. 2013; 3:272–81.14. Meignan M, Sasanelli M, Casasnovas RO, Luminari S, Fioroni F, Coriani C, et al. Metabolic tumour volumes measured at staging in lymphoma: methodological evaluation on phantom experiments and patients. Eur J Nucl Med Mol Imaging. 2014; 41:1113–22.
Article15. Sasanelli M, Meignan M, Haioun C, Berriolo-Riedinger A, Casasnovas RO, Biggi A, et al. Pretherapy metabolic tumour volume is an independent predictor of outcome in patients with diffuse large B-cell lymphoma. Eur J Nucl Med Mol Imaging. 2014; 41:2017–22.
Article16. Cottereau AS, Lanic H, Mareschal S, Meignan M, Vera P, Tilly H, et al. Molecular profile and FDG-PET/CT total metabolic tumor volume improve risk classification at diagnosis for patients with diffuse large B-cell lymphoma. Clin Cancer Res. 2016; 22:3801–9.
Article17. Cottereau AS, Hapdey S, Chartier L, Modzelewski R, Casasnovas O, Itti E, et al. Baseline total metabolic tumor volume measured with fixed or different adaptive thresholding methods equally predicts outcome in peripheral T cell lymphoma. J Nucl Med. 2017; 58:276–81.
Article18. Cheson BD, Kostakoglu L. FDG-PET for early response assessment in lymphomas: part 1-Hodgkin lymphoma. Oncology (Williston Park). 2017; 31:45–9.19. Cheson BD, Kostakoglu L. FDG-PET for early response assessment in lymphomas: part 2-diffuse large B-cell lymphoma, use of quantitative PET evaluation. Oncology (Williston Park). 2017; 31:71–6.20. Meignan M, Cottereau AS, Versari A, Chartier L, Dupuis J, Boussetta S, et al. Baseline metabolic tumor volume predicts outcome in high-tumor-burden follicular lymphoma: a pooled analysis of three multicenter studies. J Clin Oncol. 2016; 34:3618–26.
Article21. Sabattini E, Bacci F, Sagramoso C, Pileri SA. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: an overview. Pathologica. 2010; 102:83–7.22. Tohda S. Overview of lymphoid neoplasms in the fourth edition of the WHO classification. Rinsho Byori. 2012; 60:560–4.23. Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007; 25:579–86.
Article24. Juweid ME, Stroobants S, Hoekstra OS, Mottaghy FM, Dietlein M, Guermazi A, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007; 25:571–8.
Article25. Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000; 56:337–44.
Article26. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988; 44:837–45.
Article27. Kim CY, Hong CM, Kim DH, Son SH, Jeong SY, Lee SW, et al. Prognostic value of whole-body metabolic tumour volume and total lesion glycolysis measured on 18F-FDG PET/CT in patients with extranodal NK/T-cell lymphoma. Eur J Nucl Med Mol Imaging. 2013; 40:1321–9.
Article28. Ceriani L, Martelli M, Zinzani PL, Ferreri AJ, Botto B, Stelitano C, et al. Utility of baseline 18FDG-PET/CT functional parameters in defining prognosis of primary mediastinal (thymic) large B-cell lymphoma. Blood. 2015; 126:950–6.
Article29. Meignan M. Quantitative FDG-PET: a new biomarker in PMBCL. Blood. 2015; 126:924–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Application of Quantitative Indexes of FDG PET to Treatment Response Evaluation in Indolent Lymphoma
- Prognostic value of metabolic tumor volume and total lesion glycolysis from ¹â¸F-FDG PET/CT in lymph node metastases and risk stratification of endometrial carcinoma
- FDG PET/CT Maximum Tumor Dissemination to Predict Recurrence in Patients with Diffuse Large B‑Cell Lymphoma
- Baseline Total Metabolic Tumor Volume and Total Lesion Glycolysis Measured on 18F-FDG PET-CT Predict Outcomes in T-Cell Lymphoblastic Lymphoma
- Prognostic Value of Metabolic Information in Advanced Gastric Cancer Using Preoperative ¹â¸F-FDG PET/CT