J Gynecol Oncol.
2019 Nov;30(6):e89. 10.3802/jgo.2019.30.e89.
Prognostic value of metabolic tumor volume and total lesion glycolysis from ¹â¸F-FDG PET/CT in lymph node metastases and risk stratification of endometrial carcinoma
- Affiliations
-
- 1Department of Nuclear Medicine, Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China. chmarka@163.com
- 2Department of Radiology, FUWAI Central China Cardiovascular Hospital, Zhengzhou, China.
- 3Department of Gynecology, Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China.
- 4Department of Radiology, University of Texas Southwestern Medical Center, Dallas, TX, USA.
- KMID: 2458896
- DOI: http://doi.org/10.3802/jgo.2019.30.e89
Abstract
OBJECTIVE
To investigate the prognostic value of metabolic tumor volume (MTV) and total lesion glycolysis (TLG), measured by preoperative ¹â¸F-fluorodeoxyglucose positron emission tomography/computed tomography (¹â¸F-FDG PET/CT), in risk stratification of patients with endometrial carcinoma (EC).
METHODS
The patients with pathological diagnosis of EC who underwent preoperative ¹â¸F-FDG PET/CT imaging were retrospectively selected for analysis of the prognostic values of PET parameters in risk classification and lymph node metastases (LNMs). Receiver-operating-characteristic analysis was used to analyze the correlation of PET parameters cutoff values with deep myometrial invasion (MI), lymphovascular space involvement and LNM for prognostic values in risk stratification.
RESULTS
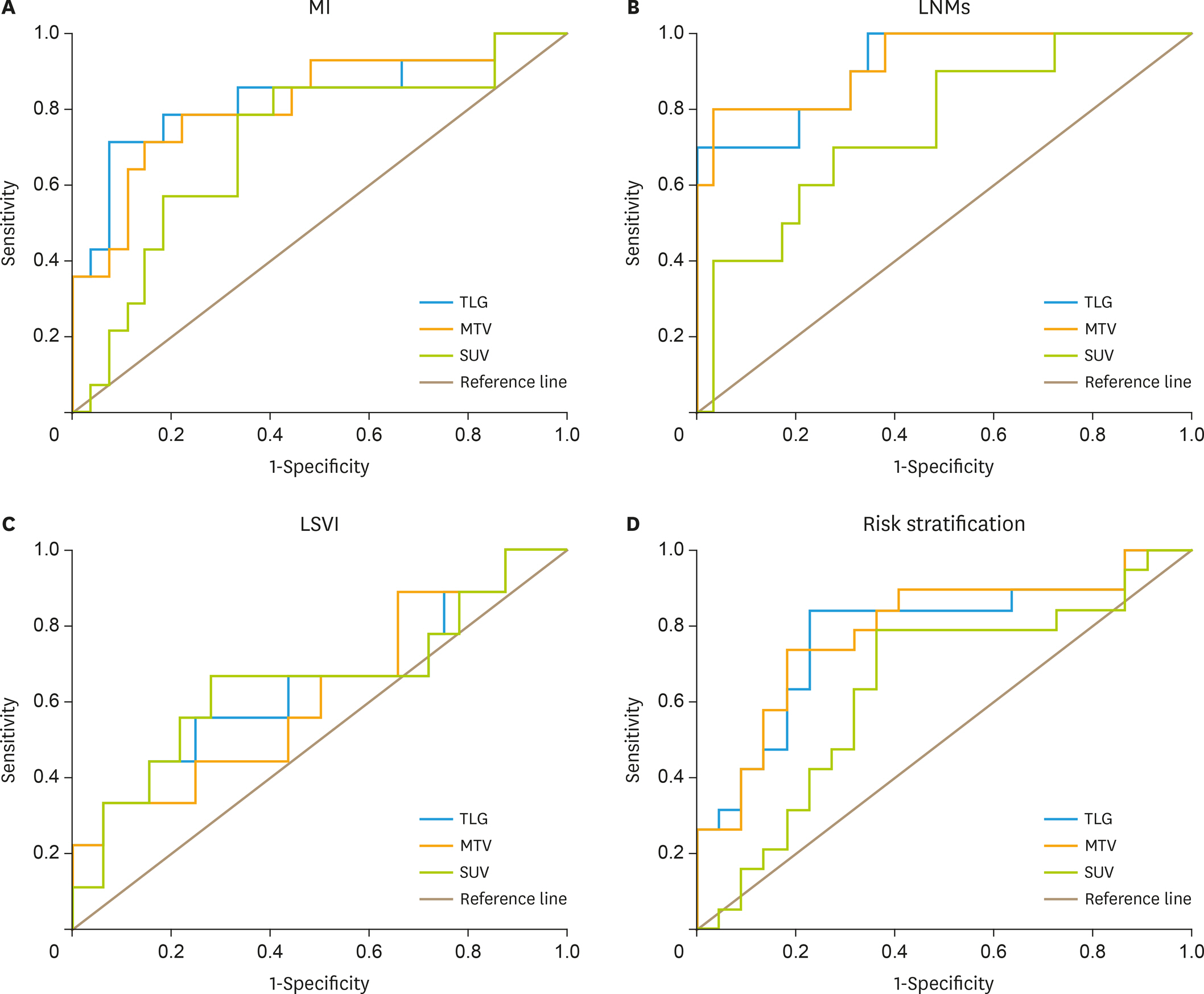
The sensitivity, specificity, positive predictive value, negative predictive value and accuracy for detection of LNM are 83.3%, 99.7%, 90.9%, 99.5% and 99.2%, respectively. The MTV and TLG of primary lesion of EC in the patients with LNM are notably higher than those in patients without LNM, p<0.010. The MTV and TLG of the EC primary lesions in high-risk patients are significantly higher than those in low-risk patients (p<0.010), but the maximum standardized uptake value (SUVmax) is not. The MTV and TLG of primary lesions were superior to SUVmax for predicting of deep MI, LNM and high-risk of EC (p<0.005).
CONCLUSION
MTV and TLG of primary lesions are more valuable in predicting risk stratification of EC patients. Preoperative ¹â¸F-FDG PET/CT imaging is useful in predicting the LNM of EC and may help guide pelvic lymphadenectomy to avoid unnecessary pelvic lymphadenectomy in EC patients with low-risk stratification.
MeSH Terms
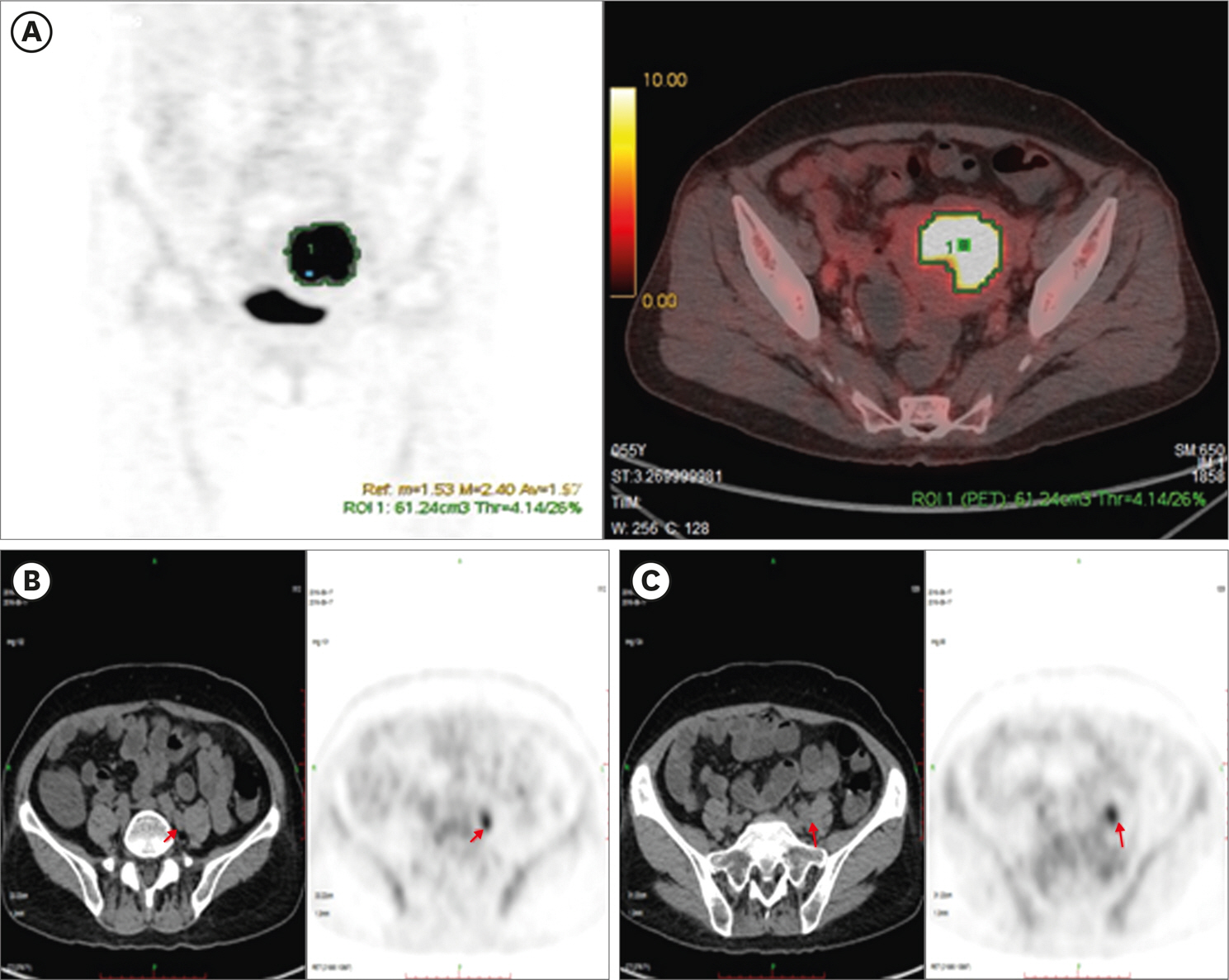
Figure
Reference
-
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016; 66:7–30.
Article2. Tirumani SH, Shanbhogue AK, Prasad SR. Current concepts in the diagnosis and management of endometrial and cervical carcinomas. Radiol Clin North Am. 2013; 51:1087–110.
Article3. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016; 66:115–32.
Article4. Boyraz G, Salman MC, Gultekin M, Basaran D, Cagan M, Ozgul N, et al. Incidence of lymph node metastasis in surgically staged FIGO Ia G1/G2 endometrial cancer with a tumor size of more than 2 cm. Int J Gynecol Cancer. 2017; 27:486–92.
Article5. Kitajima K, Suenaga Y, Ueno Y, Maeda T, Ebina Y, Yamada H, et al. Preoperative risk stratification using metabolic parameters of 18 F-FDG PET/CT in patients with endometrial cancer. Eur J Nucl Med Mol Imaging. 2015; 42:1268–75.6. Lindqvist E, Wedin M, Fredrikson M, Kjølhede P. Lymphedema after treatment for endometrial cancer – A review of prevalence and risk factors. Eur J Obstet Gynecol Reprod Biol. 2017; 211:112–21.
Article7. Lee HJ, Lee JJ, Park JY, Kim JH, Kim YM, Kim YT, et al. Prognostic value of metabolic parameters determined by preoperative 18 F-FDG PET/CT in patients with uterine carcinosarcoma. J Gynecol Oncol. 2017; 28:e43.
Article8. Atri M, Zhang Z, Dehdashti F, Lee SI, Marques H, Ali S, et al. Utility of PET/CT to evaluate retroperitoneal lymph node metastasis in high-risk endometrial cancer: results of ACRIN 6671/GOG 0233 trial. Radiology. 2017; 283:450–9.9. Pulman KJ, Dason ES, Philp L, Bernardini MQ, Ferguson SE, Laframboise S, et al. Comparison of three surgical approaches for staging lymphadenectomy in high-risk endometrial cancer. Int J Gynaecol Obstet. 2017; 136:315–9.
Article10. Todo Y, Takeshita S, Okamoto K, Yamashiro K, Kato H. Implications of paraaortic lymph node metastasis in patients with endometrial cancer without pelvic lymph node metastasis. J Gynecol Oncol. 2017; 28:e59.
Article11. Kikuchi A, Yanase T, Sasagawa M, Honma S. The role of paraaortic lymphadenectomy in stage IIIC endometrial cancer: a single-institute study. J Obstet Gynaecol. 2017; 37:510–3.
Article12. Lee HJ, Ahn BC, Hong CM, Song BI, Kim HW, Kang S, et al. Preoperative risk stratification using 18 F-FDG PET/CT in women with endometrial cancer. Nucl Med (Stuttg). 2011; 50:204–13.13. Bae HS, Lim MC, Lee JS, Lee Y, Nam BH, Seo SS, et al. Postoperative lower extremity edema in patients with primary endometrial cancer. Ann Surg Oncol. 2016; 23:186–95.
Article14. Achouri A, Huchon C, Bats AS, Bensaid C, Nos C, Lécuru F. Complications of lymphadenectomy for gynecologic cancer. Eur J Surg Oncol. 2013; 39:81–6.
Article15. Zhang C, Wang C, Feng W. Clinicopathological risk factors for pelvic lymph node metastasis in clinical early-stage endometrioid endometrial adenocarcinoma. Int J Gynecol Cancer. 2012; 22:1373–7.
Article16. Winer I, Ahmed QF, Mert I, Bandyopadhyay S, Cote M, Munkarah AR, et al. Significance of lymphovascular space invasion in uterine serous carcinoma: what matters more; extent or presence? Int J Gynecol Pathol. 2015; 34:47–56.17. Sudo S, Hattori N, Manabe O, Kato F, Mimura R, Magota K, et al. FDG PET/CT diagnostic criteria may need adjustment based on MRI to estimate the presurgical risk of extrapelvic infiltration in patients with uterine endometrial cancer. Eur J Nucl Med Mol Imaging. 2015; 42:676–84.
Article18. Nakamura K, Hongo A, Kodama J, Hiramatsu Y. The measurement of SUVmax of the primary tumor is predictive of prognosis for patients with endometrial cancer. Gynecol Oncol. 2011; 123:82–7.
Article19. Nakamura K, Kodama J, Okumura Y, Hongo A, Kanazawa S, Hiramatsu Y. The SUVmax of 18 F-FDG PET correlates with histological grade in endometrial cancer. Int J Gynecol Cancer. 2010; 20:110–5.20. Husby JA, Reitan BC, Biermann M, Trovik J, Bjørge L, Magnussen IJ, et al. Metabolic tumor volume on 18 F-FDG PET/CT improves preoperative identification of high-risk endometrial carcinoma patients. J Nucl Med. 2015; 56:1191–8.21. Shim SH, Kim DY, Lee DY, Lee SW, Park JY, Lee JJ, et al. Metabolic tumour volume and total lesion glycolysis, measured using preoperative 18 F-FDG PET/CT, predict the recurrence of endometrial cancer. BJOG. 2014; 121:1097–106.22. Tamandl D, Ta J, Schmid R, Preusser M, Paireder M, Schoppmann SF, et al. Prognostic value of volumetric PET parameters in unresectable and metastatic esophageal cancer. Eur J Radiol. 2016; 85:540–5.
Article23. Liao S, Penney BC, Zhang H, Suzuki K, Pu Y. Prognostic value of the quantitative metabolic volumetric measurement on 18 F-FDG PET/CT in Stage IV nonsurgical small-cell lung cancer. Acad Radiol. 2012; 19:69–77.24. Sun Y, Lu P, Yu L. The volume-metabolic combined parameters from 18 F-FDG PET/CT may help predict the outcomes of cervical carcinoma. Acad Radiol. 2016; 23:605–10.25. Ghooshkhanei H, Treglia G, Sabouri G, Davoodi R, Sadeghi R. Risk stratification and prognosis determination using 18 F-FDG PET imaging in endometrial cancer patients: a systematic review and meta-analysis. Gynecol Oncol. 2014; 132:669–76.26. Wang ZQ, Wang JL, Shen DH, Li XP, Wei LH. Should all endometrioid uterine cancer patients undergo systemic lymphadenectomy? Eur J Surg Oncol. 2013; 39:344–9.
Article27. Mariani A, Webb MJ, Keeney GL, Haddock MG, Calori G, Podratz KC. Low-risk corpus cancer: is lymphadenectomy or radiotherapy necessary? Am J Obstet Gynecol. 2000; 182:1506–19.
Article28. Antonsen SL, Jensen LN, Loft A, Berthelsen AK, Costa J, Tabor A, et al. MRI, PET/CT and ultrasound in the preoperative staging of endometrial cancer – a multicenter prospective comparative study. Gynecol Oncol. 2013; 128:300–8.
Article29. Signorelli M, Crivellaro C, Buda A, Guerra L, Fruscio R, Elisei F, et al. Staging of high-risk endometrial cancer with PET/CT and sentinel lymph node mapping. Clin Nucl Med. 2015; 40:780–5.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Neck CT and ¹â¸F-FDG PET-CT for Making the Preoperative Diagnosis of Lymph Node Metastasis in Papillary Thyroid Cancer
- Imaging of Gastric Cancer Metabolism Using 18 F-FDG PET/CT
- Prognostic Value of Metabolic Information in Advanced Gastric Cancer Using Preoperative ¹â¸F-FDG PET/CT
- Significance of ¹â¸F FDG PET-CT for Preoperative Diagnosis of Cervical Lymph Nodes Metastasis in Papillary Thyroid Carinoma
- Focal Nasopharyngeal Activity Detected on [18F]FDG PET/CT: Clinical Implications and Comparison of Metabolic Parameters for Prediction of Malignancy