Cancer Res Treat.
2019 Oct;51(4):1464-1478. 10.4143/crt.2018.657.
Novel Prognostic Nomograms Based on Inflammation-Related Markers for Patients with Hepatocellular Carcinoma Underwent Hepatectomy
- Affiliations
-
- 1Department of Liver Surgery, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China.
- 2Department of Gastrointestinal Surgery, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China.
- 3Department of Medical Imaging, Sun Yat-sen University Cancer Center, Guangzhou, China.
- 4Clinical Trials Unit, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China. pengsui@vip.163.com
- 5Division of Interventional Ultrasound, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China.
- 6Department of Pathology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China. caoqhua@mail.sysu.edu.cn
- 7Department of Gastroenterology and Hepatology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China.
- KMID: 2460595
- DOI: http://doi.org/10.4143/crt.2018.657
Abstract
- PURPOSE
Hepatocellular carcinoma (HCC) is an aggressive disease with high recurrence rate. However, current staging systems were lack of predictive capacity for HCC recurrence. We aimed to develop prognostic nomograms based on inflammation-related markers for HCC patients underwent hepatectomy.
MATERIALS AND METHODS
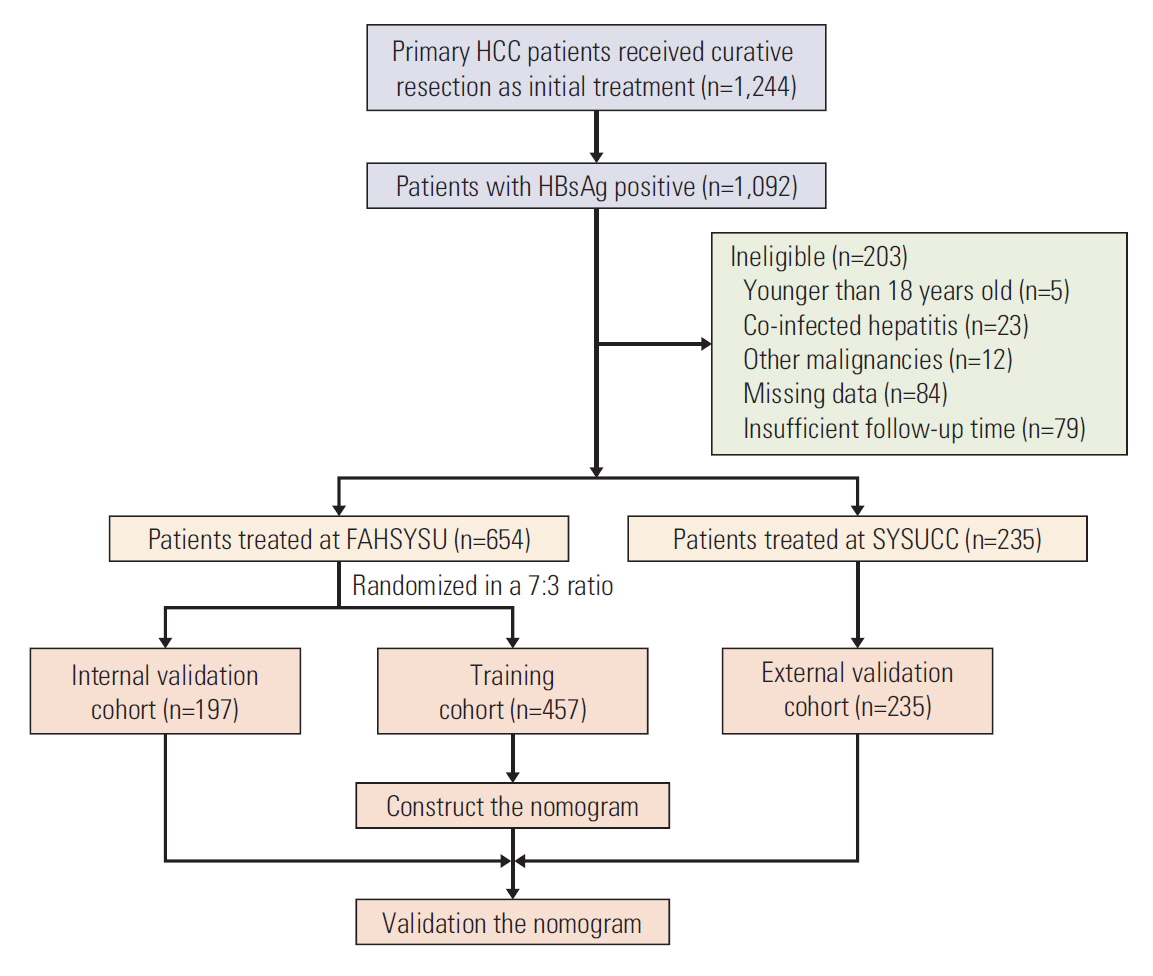
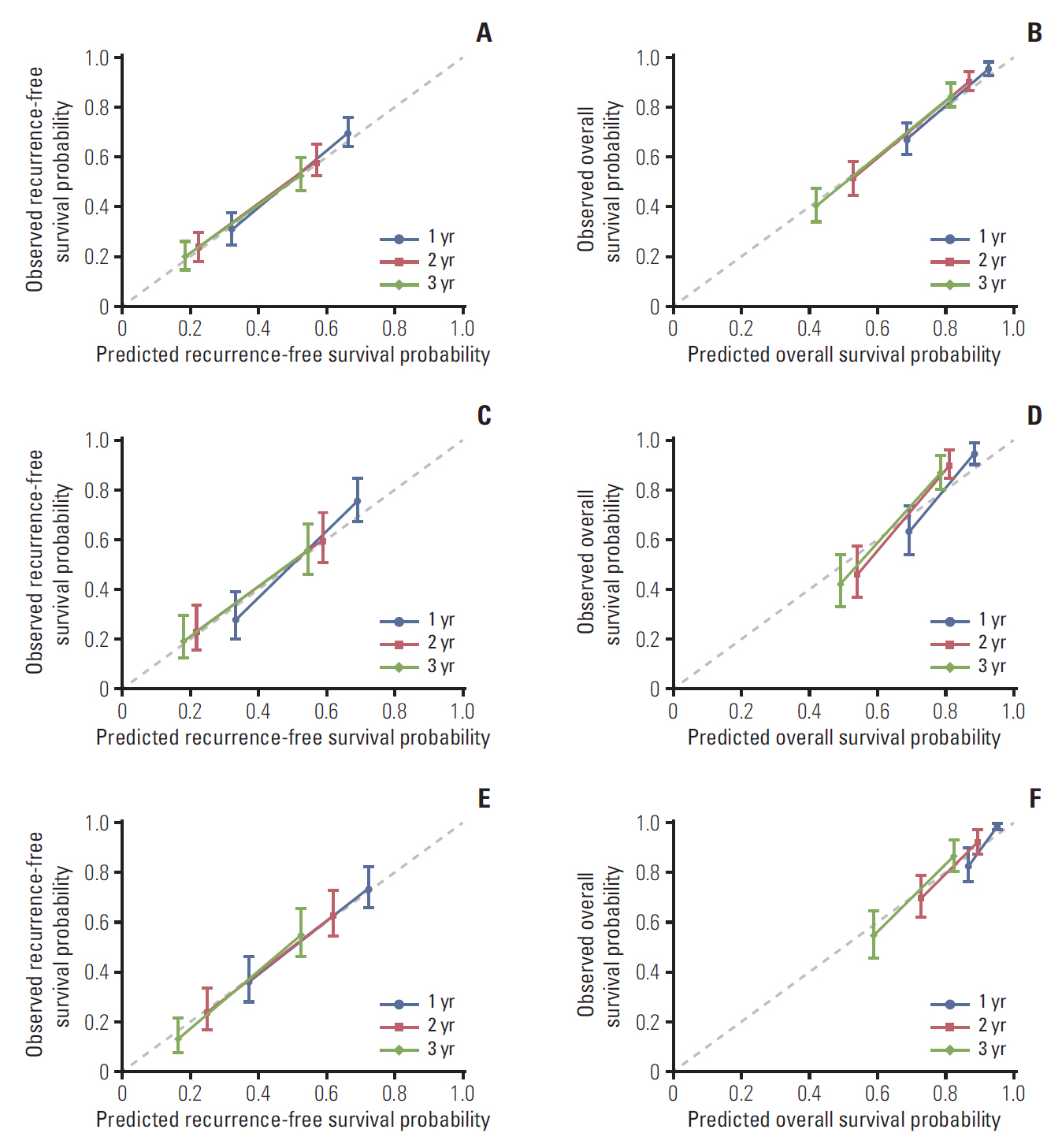
We recruited 889 surgically treated patients from two medical centers. Independent prognostic factors were identified by cox regression analyses. Nomograms for recurrence-free survival (RFS) and overall survival (OS) were established, and validated internally and externally. The performance, discrimination, and calibration of nomograms were assessed, and compared with existed staging systems.
RESULTS
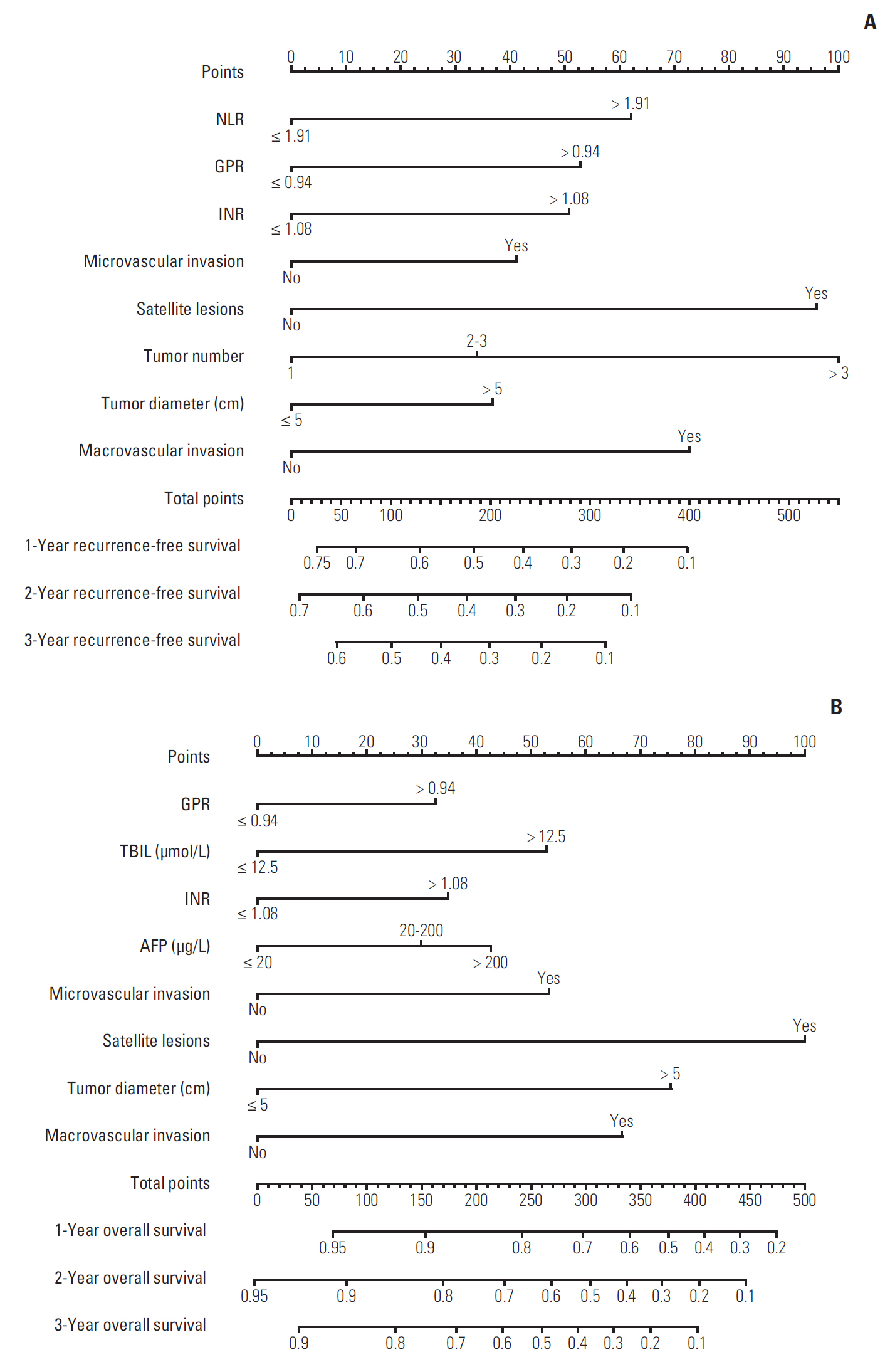
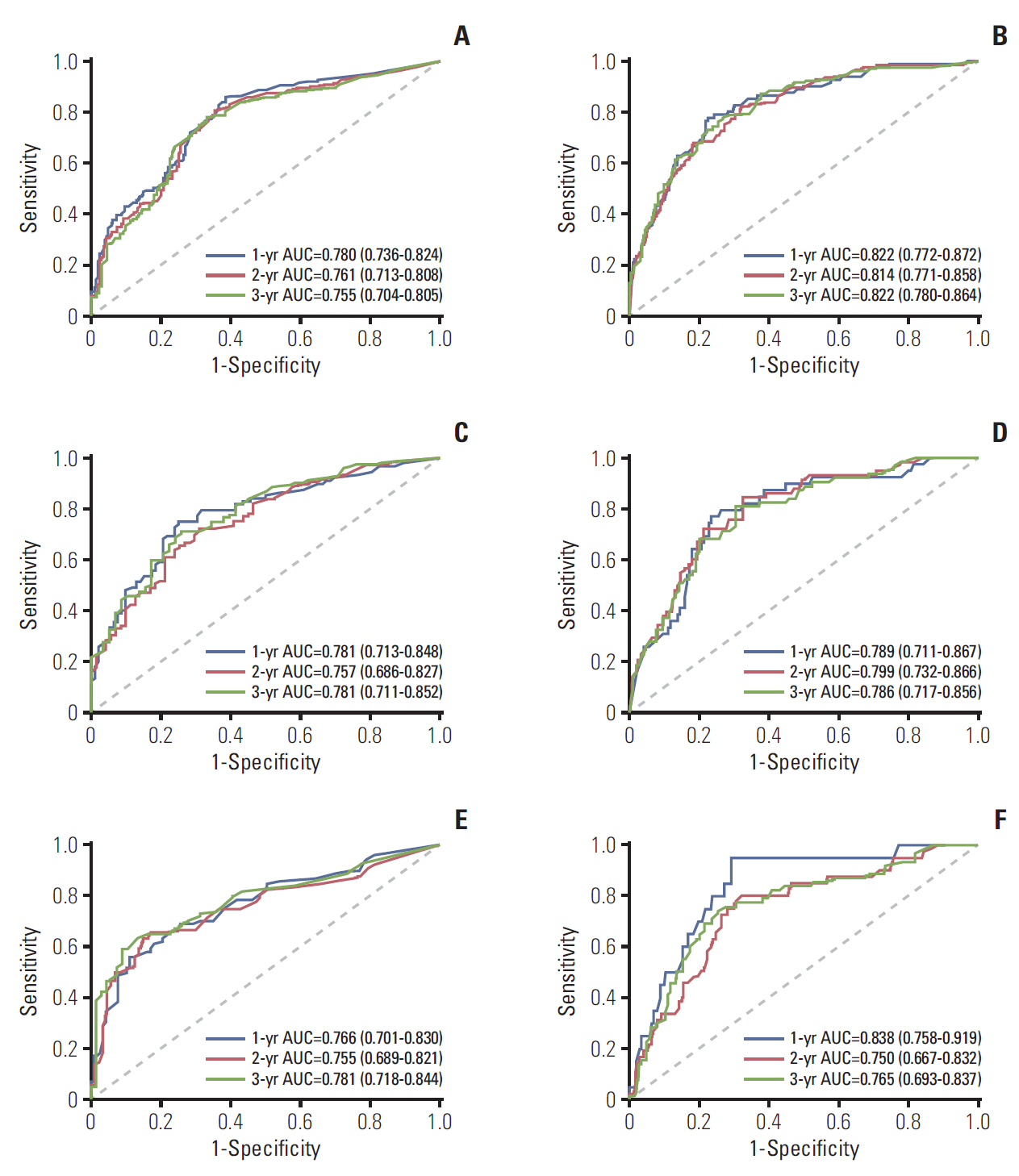
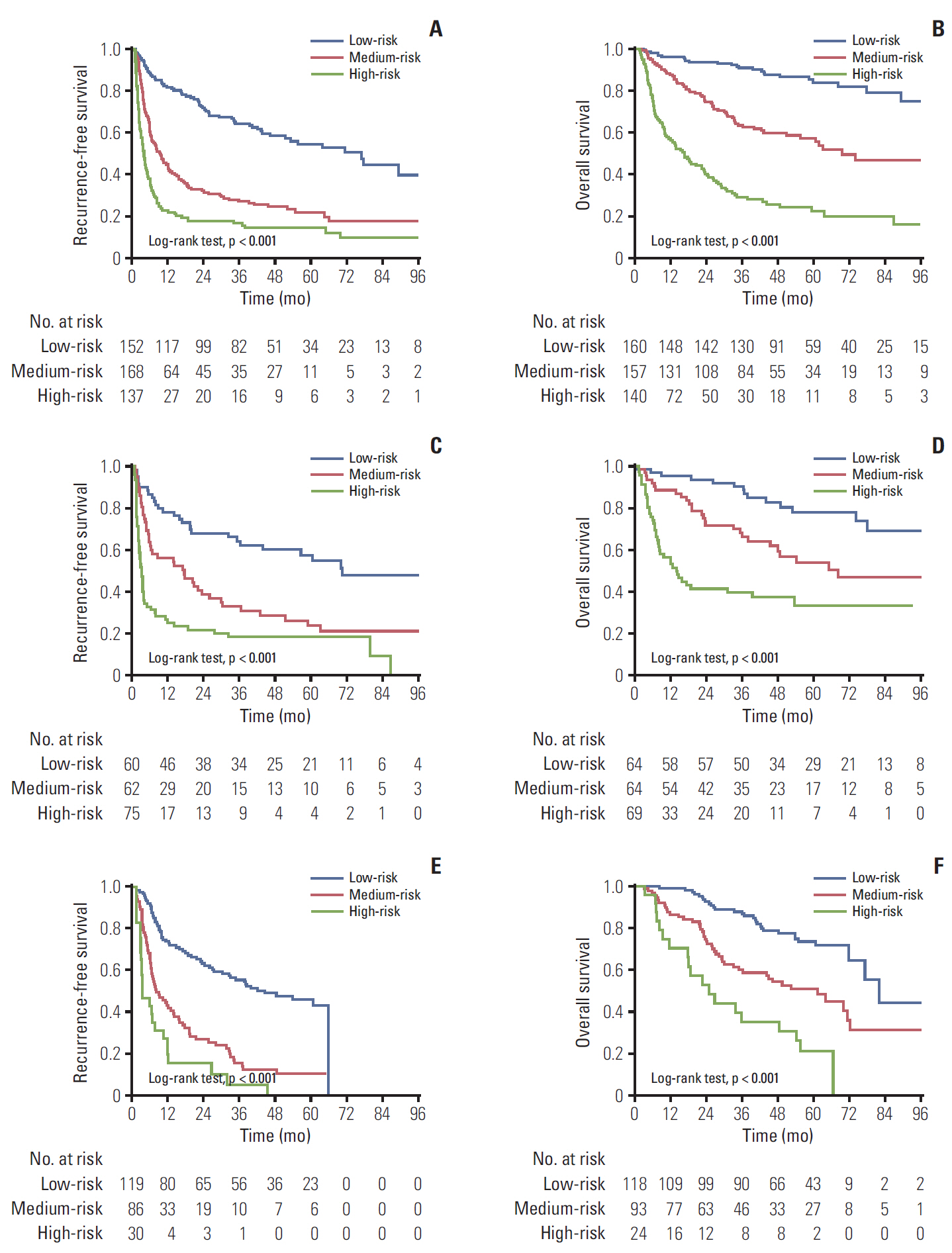
Neutrophil to lymphocyte ratio (NLR) and gamma-glutamyl transpeptidase to platelet ratio (GPR) were the two inflammation-related factor that independently correlated with survival. NLR, GPR, international normalized ratio (INR), microvascular invasion, satellite lesions, tumour number, tumour diameter, and macrovascular invasion were used to construct nomogram for RFS while GPR, total bilirubin, INR, α-fetoprotein, microvascular invasion, satellite lesions, tumour diameter, and macrovascular invasion were for OS. In the training cohort, the C-index of nomogram was 0.701 (95% confidence interval [CI], 0.669 to 0.732) for RFS and 0.761 (95% CI, 0.728 to 0.795) for OS. These results received both internal and external validation with C-index of 0.701 (95% CI, 0.647 to 0.755) and 0.707 (95% CI, 0.657 to 0.756) for RFS, and 0.706 (95% CI, 0.640 to 0.772) and 0.708 (95% CI, 0.646 to 0.771) for OS, respectively. The nomograms showed superior accuracy to conventional staging systems (p<0.001).
CONCLUSION
The nomograms based on inflammation-related markers are of high efficacy in predicting survival of HCC patients after hepatectomy, which will be valuable in guiding postoperative interventions and follow-ups.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424.
Article2. Portolani N, Coniglio A, Ghidoni S, Giovanelli M, Benetti A, Tiberio GA, et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006; 243:229–35.3. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018; 69:182–236.4. de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012; 13:607–15.
Article5. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008; 454:436–44.
Article6. Trepo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014; 384:2053–63.7. Berasain C, Castillo J, Perugorria MJ, Latasa MU, Prieto J, Avila MA. Inflammation and liver cancer: new molecular links. Ann N Y Acad Sci. 2009; 1155:206–21.8. Sasaki A, Iwashita Y, Shibata K, Matsumoto T, Ohta M, Kitano S. Prognostic value of preoperative peripheral blood monocyte count in patients with hepatocellular carcinoma. Surgery. 2006; 139:755–64.
Article9. Gomez D, Farid S, Malik HZ, Young AL, Toogood GJ, Lodge JP, et al. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J Surg. 2008; 32:1757–62.
Article10. Smith RA, Bosonnet L, Raraty M, Sutton R, Neoptolemos JP, Campbell F, et al. Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am J Surg. 2009; 197:466–72.
Article11. Lin ZX, Ruan DY, Li Y, Wu DH, Ma XK, Chen J, et al. Lymphocyte-to-monocyte ratio predicts survival of patients with hepatocellular carcinoma after curative resection. World J Gastroenterol. 2015; 21:10898–906.
Article12. Wang WL, Zheng XL, Zhang ZY, Zhou Y, Hao J, Tang G, et al. Preoperative gamma-glutamyl transpeptidase to platelet ratio (GPR) is an independent prognostic factor for HBV-related hepatocellular carcinoma after curative hepatic resection. Medicine (Baltimore). 2016; 95:e4087.13. Chan AW, Chan SL, Wong GL, Wong VW, Chong CC, Lai PB, et al. Prognostic Nutritional Index (PNI) predicts tumor recurrence of very early/early stage hepatocellular carcinoma after surgical resection. Ann Surg Oncol. 2015; 22:4138–48.
Article14. Yang Z, Zhang J, Lu Y, Xu Q, Tang B, Wang Q, et al. Aspartate aminotransferase-lymphocyte ratio index and systemic immune-inflammation index predict overall survival in HBV-related hepatocellular carcinoma patients after transcatheter arterial chemoembolization. Oncotarget. 2015; 6:43090–8.
Article15. Shen SL, Fu SJ, Chen B, Kuang M, Li SQ, Hua YP, et al. Preoperative aspartate aminotransferase to platelet ratio is an independent prognostic factor for hepatitis B-induced hepatocellular carcinoma after hepatic resection. Ann Surg Oncol. 2014; 21:3802–9.
Article16. Ji F, Fu S, Guo Z, Pang H, Chen D, Wang X, et al. Prognostic significance of preoperative aspartate aminotransferase to neutrophil ratio index in patients with hepatocellular carcinoma after hepatic resection. Oncotarget. 2016; 7:72276–89.
Article17. Pinato DJ, Stebbing J, Ishizuka M, Khan SA, Wasan HS, North BV, et al. A novel and validated prognostic index in hepatocellular carcinoma: the inflammation based index (IBI). J Hepatol. 2012; 57:1013–20.
Article18. Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014; 20:6212–22.
Article19. Shim JH, Jun MJ, Han S, Lee YJ, Lee SG, Kim KM, et al. Prognostic nomograms for prediction of recurrence and survival after curative liver resection for hepatocellular carcinoma. Ann Surg. 2015; 261:939–46.
Article20. Yang P, Qiu J, Li J, Wu D, Wan X, Lau WY, et al. Nomograms for pre- and postoperative prediction of long-term survival for patients who underwent hepatectomy for multiple hepatocellular carcinomas. Ann Surg. 2016; 263:778–86.
Article21. Shen J, He L, Li C, Wen T, Chen W, Lu C, et al. Prognostic nomograms for patients with resectable hepatocelluar carcinoma incorporating systemic inflammation and tumor characteristics. Oncotarget. 2016; 7:80783–93.
Article22. He W, Peng B, Tang Y, Yang J, Zheng Y, Qiu J, et al. Nomogram to predict survival of patients with recurrence of hepatocellular carcinoma after surgery. Clin Gastroenterol Hepatol. 2018; 16:756–64. e10.
Article23. Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg. 2005; 12:351–5.
Article24. Shi J, Lai EC, Li N, Guo WX, Xue J, Lau WY, et al. Surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol. 2010; 17:2073–80.
Article25. European Association For the Study of the Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012; 56:908–43.26. Pinato DJ, North BV, Sharma R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI). Br J Cancer. 2012; 106:1439–45.
Article27. Lemoine M, Shimakawa Y, Nayagam S, Khalil M, Suso P, Lloyd J, et al. The gamma-glutamyl transpeptidase to platelet ratio (GPR) predicts significant liver fibrosis and cirrhosis in patients with chronic HBV infection in West Africa. Gut. 2016; 65:1369–76.
Article28. Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003; 38:200–7.
Article29. Kluger MD, Salceda JA, Laurent A, Tayar C, Duvoux C, Decaens T, et al. Liver resection for hepatocellular carcinoma in 313 Western patients: tumor biology and underlying liver rather than tumor size drive prognosis. J Hepatol. 2015; 62:1131–40.
Article30. Zhong JH, Ke Y, Gong WF, Xiang BD, Ma L, Ye XP, et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg. 2014; 260:329–40.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prognostic Significance of Preoperative Controlling Nutritional Status Score in Patients Who Underwent Hepatic Resection for Hepatocellular Carcinoma
- Prognostic Factors and Clinicopathologic Features after Resection of Small Hepatocellular Carcinoma (< or =2 cm)
- Nomograms to Predict the Individual Survival of Patients with Solitary Hepatocellular Carcinoma after Hepatectomy
- Two-Stage Hepatectomy for Bilateral Hepatocellular Carcinoma with Bile Duct Tumor Thrombi
- The risk factors of early recurrence after hepatectomy in hepatocellular carcinoma