Cancer Res Treat.
2019 Oct;51(4):1302-1312. 10.4143/crt.2018.555.
Body Cavity–Based Lymphoma in a Country with Low Human Immunodeficiency Virus Prevalence: A Series of 17 Cases from the Consortium for Improving Survival of Lymphoma
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea. go01@snu.ac.kr
- 2Department of Pathology, Samsung Medical Center, Seoul, Korea.
- 3Department of Internal Medicine, Dong-A University College of Medicine, Busan, Korea.
- 4Department of Oncology, Asan Medical Center, Seoul, Korea.
- 5Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- 6Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 7Department of Internal Medicine, Korea University Anam Hospital, Seoul, Korea.
- 8Department of Internal Medicine, Pusan National University Hospital, Busan, Korea.
- 9Department of Medicine, Samsung Medical Center, Seoul, Korea. wskimsmc@skku.edu
- 10Department of Internal Medicine, Soonchunhyang University Seoul Hospital, Seoul, Korea.
- KMID: 2460580
- DOI: http://doi.org/10.4143/crt.2018.555
Abstract
- PURPOSE
Primary effusion lymphoma (PEL) is a type of body cavity-based lymphoma (BCBL). Most patients with PEL are severely immunocompromised and seropositive for human immunodeficiency virus (HIV). We investigated the distinctive clinicopathologic characteristics of BCBL in a country with low HIV burden.
MATERIALS AND METHODS
We retrospectively collected data on the clinicopathologic characteristics, treatments, and outcomes of 17 consecutive patients with BCBL at nine institutions in Korea.
RESULTS
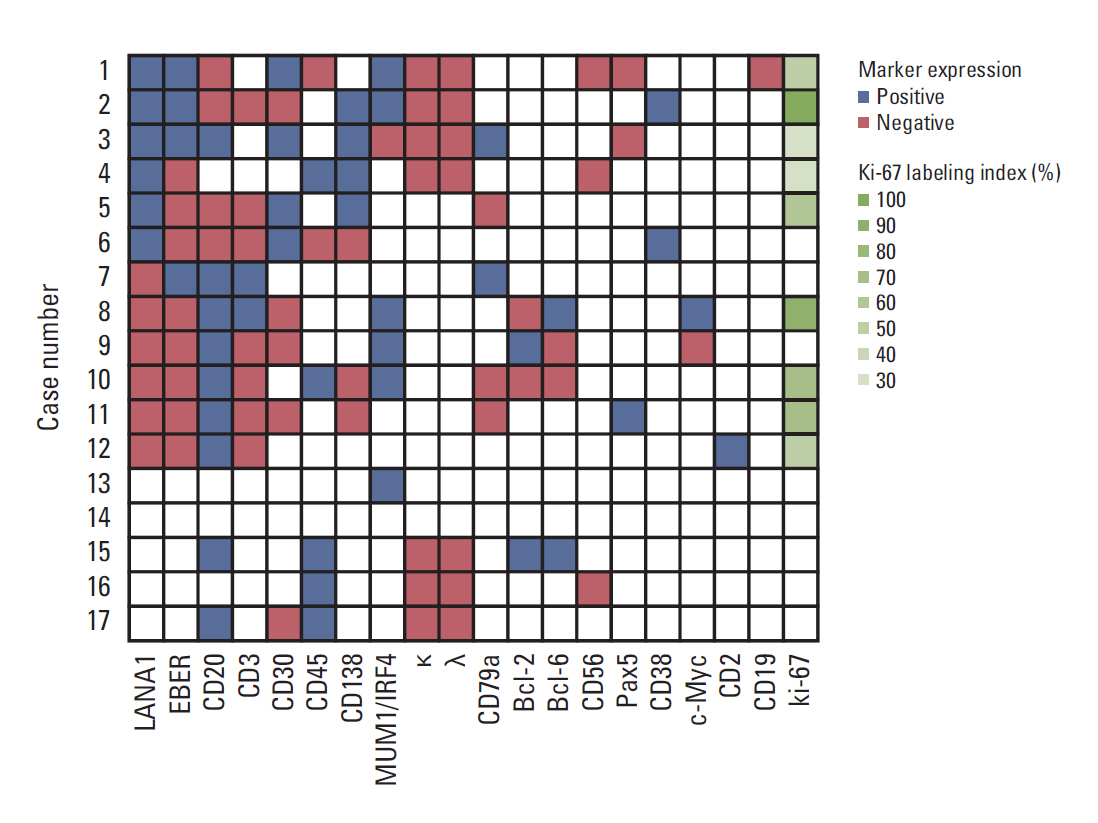
Latency-associated nuclear antigen 1 (LANA1) immunostaining indicated that six patients had PEL, six patients had human herpesvirus 8 (HHV8)-unrelated BCBL, and five patients had HHV8-unknown BCBL. The patients with PEL exhibited no evidence of immunodeficiency except for one who was HIV positive. One (20%) and four (80%) patients with PEL and six (100%) and zero (0%) patients with HHV8-unrelated BCBL were positive for CD20 and CD30 expression, respectively. The two patients with PEL (one HIV-positive and one HIV-negative patient) with the lowest proliferation activity as assessed by the Ki-67 labeling index survived for > 1 and > 4 years without chemotherapy, respectively, in contrast to the PEL cases in the literature, which mostly showed a high proliferation index and poor survival.
CONCLUSION
PEL mostly occurred in ostensibly immunocompetent individuals and had a favorable outcome in Korea. A watchful waiting approach may be applicable for managing HIV-seronegative patients with PEL with a low Ki-67 labeling index. A possible trend was detected among LANA1, CD20, and CD30 expression in BCBL.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Chen YB, Rahemtullah A, Hochberg E. Primary effusion lymphoma. Oncologist. 2007; 12:569–76.
Article2. Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995; 332:1186–91.
Article3. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016; 127:2375–90.
Article4. Nador RG, Cesarman E, Chadburn A, Dawson DB, Ansari MQ, Sald J, et al. Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi's sarcomaassociated herpes virus. Blood. 1996; 88:645–56.
Article5. Boulanger E, Agbalika F, Maarek O, Daniel MT, Grollet L, Molina JM, et al. A clinical, molecular and cytogenetic study of 12 cases of human herpesvirus 8 associated primary effusion lymphoma in HIV-infected patients. Hematol J. 2001; 2:172–9.
Article6. Simonelli C, Spina M, Cinelli R, Talamini R, Tedeschi R, Gloghini A, et al. Clinical features and outcome of primary effusion lymphoma in HIV-infected patients: a single-institution study. J Clin Oncol. 2003; 21:3948–54.
Article7. Boulanger E, Gerard L, Gabarre J, Molina JM, Rapp C, Abino JF, et al. Prognostic factors and outcome of human herpesvirus 8-associated primary effusion lymphoma in patients with AIDS. J Clin Oncol. 2005; 23:4372–80.
Article8. Guillet S, Gerard L, Meignin V, Agbalika F, Cuccini W, Denis B, et al. Classic and extracavitary primary effusion lymphoma in 51 HIV-infected patients from a single institution. Am J Hematol. 2016; 91:233–7.
Article9. Said JW, Tasaka T, Takeuchi S, Asou H, de Vos S, Cesarman E, et al. Primary effusion lymphoma in women: report of two cases of Kaposi's sarcoma herpes virus-associated effusion-based lymphoma in human immunodeficiency virus-negative women. Blood. 1996; 88:3124–8.
Article10. Jones D, Ballestas ME, Kaye KM, Gulizia JM, Winters GL, Fletcher J, et al. Primary-effusion lymphoma and Kaposi's sarcoma in a cardiac-transplant recipient. N Engl J Med. 1998; 339:444–9.
Article11. Boulanger E, Afonso PV, Yahiaoui Y, Adle-Biassette H, Gabarre J, Agbalika F. Human herpesvirus-8 (HHV-8)-associated primary effusion lymphoma in two renal transplant recipients receiving rapamycin. Am J Transplant. 2008; 8:707–10.
Article12. Hermine O, Michel M, Buzyn-Veil A, Gessain A. Body-cavity-based lymphoma in an HIV-seronegative patient without Kaposi's sarcoma-associated herpesvirus-like DNA sequences. N Engl J Med. 1996; 334:272–3.
Article13. Rodriguez J, Romaguera JE, Katz RL, Said J, Cabanillas F. Primary effusion lymphoma in an HIV-negative patient with no serologic evidence of Kaposi's sarcoma virus. Leuk Lymphoma. 2001; 41:185–9.
Article14. Matsumoto Y, Nomura K, Ueda K, Satoh K, Yasuda N, Taki T, et al. Human herpesvirus 8-negative malignant effusion lymphoma: a distinct clinical entity and successful treatment with rituximab. Leuk Lymphoma. 2005; 46:415–9.
Article15. Shin J, Lee JO, Choe JY, Bang SM, Lee JS. Human herpesvirus 8-unrelated primary effusion lymphoma-like lymphoma in an elderly Korean patient with a good response to rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone. Cancer Res Treat. 2017; 49:274–8.
Article16. Kobayashi Y, Kamitsuji Y, Kuroda J, Tsunoda S, Uoshima N, Kimura S, et al. Comparison of human herpes virus 8 related primary effusion lymphoma with human herpes virus 8 unrelated primary effusion lymphoma-like lymphoma on the basis of HIV: report of 2 cases and review of 212 cases in the literature. Acta Haematol. 2007; 117:132–44.
Article17. Alexanian S, Said J, Lones M, Pullarkat ST. KSHV/HHV8-negative effusion-based lymphoma, a distinct entity associated with fluid overload states. Am J Surg Pathol. 2013; 37:241–9.
Article18. Wu W, Youm W, Rezk SA, Zhao X. Human herpesvirus 8-unrelated primary effusion lymphoma-like lymphoma: report of a rare case and review of 54 cases in the literature. Am J Clin Pathol. 2013; 140:258–73.19. Hall JC, Hall BJ, Cockerell CJ. HIV/AIDS in the post-HAART era: manifestations, treatment, and epidemiology. Shelton, CT: People’s Medical Publishing House, USA;2011.20. Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007; 25:579–86.
Article21. Choe KW. Epidemiology of HIV/AIDS: current status, trend and prospect. J Korean Med Assoc. 2007; 50:296–302.22. Oksenhendler E, Clauvel JP, Jouveshomme S, Davi F, Mansour G. Complete remission of a primary effusion lymphoma with antiretroviral therapy. Am J Hematol. 1998; 57:266.
Article23. Hocqueloux L, Agbalika F, Oksenhendler E, Molina JM. Long-term remission of an AIDS-related primary effusion lymphoma with antiviral therapy. AIDS. 2001; 15:280–2.
Article24. Carbone A, Gloghini A, Bontempo D, Monini P, Tirelli U, Volpe R, et al. Proliferation in HHV-8-positive primary effusion lymphomas is associated with expression of HHV-8 cyclin but independent of p27(kip1). Am J Pathol. 2000; 156:1209–15.
Article25. Pro B, Advani R, Brice P, Bartlett NL, Rosenblatt JD, Illidge T, et al. Five-year results of brentuximab vedotin in patients with relapsed or refractory systemic anaplastic large cell lymphoma. Blood. 2017; 130:2709–17.
Article26. Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J Clin Oncol. 2012; 30:2183–9.27. Connors JM, Jurczak W, Straus DJ, Ansell SM, Kim WS, Gallamini A, et al. Brentuximab vedotin with chemotherapy for stage III or IV Hodgkin's lymphoma. N Engl J Med. 2018; 378:331–44.
Article28. Jacobsen ED, Sharman JP, Oki Y, Advani RH, Winter JN, Bello CM, et al. Brentuximab vedotin demonstrates objective responses in a phase 2 study of relapsed/refractory DLBCL with variable CD30 expression. Blood. 2015; 125:1394–402.
Article29. Bhatt S, Ashlock BM, Natkunam Y, Sujoy V, Chapman JR, Ramos JC, et al. CD30 targeting with brentuximab vedotin: a novel therapeutic approach to primary effusion lymphoma. Blood. 2013; 122:1233–42.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Primary Effusion Lymphoma in a Non-Human Immunodeficiency Virus Patient: A Case Report
- Human Herpesvirus 8-Negative and Epstein-Barr Virus-Positive Effusion-Based Lymphoma in a Patient with Human Immunodeficiency Virus
- Plasmablastic Lymphoma in the Anal Canal
- Consortium for Improving Survival of Lymphoma (CISL): a model of multicenter collaboration for lymphoma studies in Korea
- Plasmablastic Lymphoma in a Human Immunodeficiency Virus-negative Patient: A Case Report and Review of the Literature