Blood Res.
2019 Sep;54(3):218-228. 10.5045/br.2019.54.3.218.
Prognostic utility of ADAMTS13 activity for the atypical hemolytic uremic syndrome (aHUS) and comparison of complement serology between aHUS and thrombotic thrombocytopenic purpura
- Affiliations
-
- 1Department of Internal Medicine, CHA Bundang Medical Center, CHA University, Seongnam, Korea. doh@cha.ac.kr
- 2Institute for Clinical Research, School of Medicine CHA University, Seongnam, Korea.
- 3Department Laboratory Medicine, CHA Bundang Medical Center, CHA University, Seongnam, Korea.
- 4Western Australian Centre for Thrombosis and Haemostasis, Murdoch University, Perth, Australia.
- KMID: 2459388
- DOI: http://doi.org/10.5045/br.2019.54.3.218
Abstract
- BACKGROUND
Atypical hemolytic uremic syndrome (aHUS) involves dysregulation of the complement system, but whether this also occurs in thrombotic thrombocytopenic purpura (TTP) remains unclear. Although these conditions are difficult to differentiate clinically, TTP can be distinguished by low (<10%) ADAMTS13 activity. The aim was to identify the differences in complement activation products between TTP and aHUS and investigate ADAMTS13 activity as a prognostic factor in aHUS.
METHODS
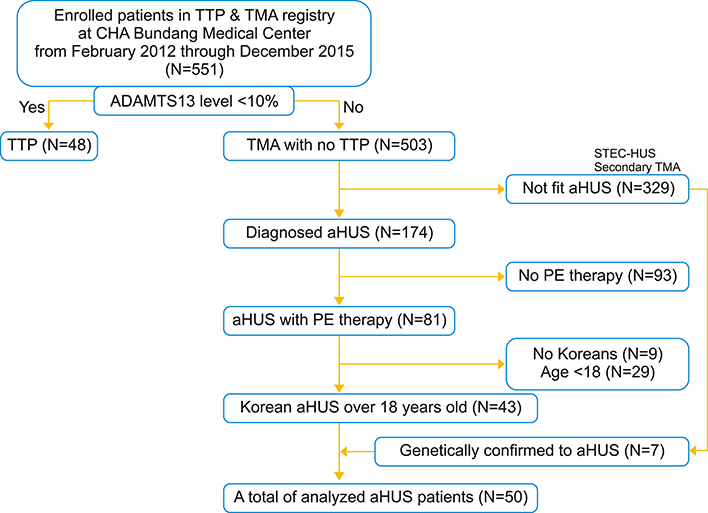
We analyzed patients with thrombotic microangiopathy diagnosed as TTP (N=48) or aHUS (N=50), selected from a Korean registry (N=551). Complement activation products in the plasma samples collected from the patients prior to treatment and in 40 healthy controls were measured by ELISA.
RESULTS
The levels of generalized (C3a), alternate (factor Bb), and terminal (C5a and C5b-9) markers were significantly higher (all P<0.01) in the patients than in the healthy controls. Only the factor Bb levels significantly differed (P=0.008) between the two disease groups. In aHUS patients, high normal ADAMTS13 activity (≥77%) was associated with improved treatment response (OR, 6.769; 95% CI, 1.605-28.542; P=0.005), remission (OR, 6.000; 95% CI, 1.693-21.262; P=0.004), exacerbation (OR, 0.242; 95% CI, 0.064-0.916; P=0.031), and disease-associated mortality rates (OR, 0.155; 95% CI, 0.029-0.813; P=0.017).
CONCLUSION
These data suggest that complement biomarkers, except factor Bb, are similarly activated in TTP and aHUS patients, and ADAMTS13 activity can predict the treatment response and outcome in aHUS patients.
Keyword
MeSH Terms
Figure
Reference
-
1. George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014; 371:1847–1848.
Article2. Mannucci PM, Cugno M. The complex differential diagnosis between thrombotic thrombocytopenic purpura and the atypical hemolytic uremic syndrome: laboratory weapons and their impact on treatment choice and monitoring. Thromb Res. 2015; 136:851–854.
Article3. Crawley JT, Scully MA. Thrombotic thrombocytopenic purpura: basic pathophysiology and therapeutic strategies. Hematology Am Soc Hematol Educ Program. 2013; 2013:292–299.
Article4. Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med. 2009; 361:1676–1687.
Article5. Loirat C, Fremeaux-Bacchi V. Atypical hemolytic uremic syndrome. Orphanet J Rare Dis. 2011; 6:60.
Article6. Afshar-Kharghan V. Atypical hemolytic uremic syndrome. Hematology Am Soc Hematol Educ Program. 2016; 2016:217–225.
Article7. Ruiz-Torres MP, Casiraghi F, Galbusera M, et al. Complement activation: the missing link between ADAMTS-13 deficiency and microvascular thrombosis of thrombotic microangiopathies. Thromb Haemost. 2005; 93:443–452.
Article8. Jang MJ, Chong SY, Kim IH, et al. Clinical features of severe acquired ADAMTS13 deficiency in thrombotic thrombocytopenic purpura: the Korean TTP registry experience. Int J Hematol. 2011; 93:163–169.
Article9. Cataland SR, Holers VM, Geyer S, Yang S, Wu HM. Biomarkers of terminal complement activation confirm the diagnosis of aHUS and differentiate aHUS from TTP. Blood. 2014; 123:3733–3738.
Article10. Coppo P, Schwarzinger M, Buffet M, et al. Predictive features of severe acquired ADAMTS13 deficiency in idiopathic thrombotic microangiopathies: the French TMA reference center experience. PLoS One. 2010; 5:e10208.
Article11. Vesely SK, George JN, Lammle B, et al. ADAMTS13 activity in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: relation to presenting features and clinical outcomes in a prospective cohort of 142 patients. Blood. 2003; 102:60–68.
Article12. Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010; 11:785–797.
Article13. Noris M, Mescia F, Remuzzi G. STEC-HUS, atypical HUS and TTP are all diseases of complement activation. Nat Rev Nephrol. 2012; 8:622–633.
Article14. Kavanagh D, Goodship TH. Atypical hemolytic uremic syndrome, genetic basis, and clinical manifestations. Hematology Am Soc Hematol Educ Program. 2011; 2011:15–20.
Article15. Legendre CM, Licht C, Muus P, et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013; 368:2169–2181.
Article16. Kremer Hovinga JA, Vesely SK, Terrell DR, Lammle B, George JN. Survival and relapse in patients with thrombotic thrombocytopenic purpura. Blood. 2010; 115:1500–1511.
Article17. Reti M, Farkas P, Csuka D, et al. Complement activation in thrombotic thrombocytopenic purpura. J Thromb Haemost. 2012; 10:791–798.
Article18. Westwood JP, Langley K, Heelas E, Machin SJ, Scully M. Complement and cytokine response in acute thrombotic thrombocytopenic purpura. Br J Haematol. 2014; 164:858–866.
Article19. Wu TC, Yang S, Haven S, et al. Complement activation and mortality during an acute episode of thrombotic thrombocytopenic purpura. J Thromb Haemost. 2013; 11:1925–1927.
Article20. Feng S, Liang X, Kroll MH, Chung DW, Afshar-Kharghan V. von Willebrand factor is a cofactor in complement regulation. Blood. 2015; 125:1034–1037.
Article21. Turner NA, Moake J. Assembly and activation of alternative complement components on endothelial cell-anchored ultra-large von Willebrand factor links complement and hemostasisthrombosis. PLoS One. 2013; 8:e59372.
Article22. Thurman JM, Marians R, Emlen W, et al. Alternative pathway of complement in children with diarrhea-associated hemolytic uremic syndrome. Clin J Am Soc Nephrol. 2009; 4:1920–1924.
Article23. Martin K, Borgel D, Lerolle N, et al. Decreased ADAMTS-13 (A disintegrin-like and metalloprotease with thrombospondin type 1 repeats) is associated with a poor prognosis in sepsis-induced organ failure. Crit Care Med. 2007; 35:2375–2382.
Article24. Farkas P, Csuka D, Mikes B, et al. Complement activation, inflammation and relative ADAMTS13 deficiency in secondary thrombotic microangiopathies. Immunobiology. 2017; 222:119–127.
Article25. Khanal N, Dahal S, Upadhyay S, Bhatt VR, Bierman PJ. Differentiating malignant hypertension-induced thrombotic microangiopathy from thrombotic thrombocytopenic purpura. Ther Adv Hematol. 2015; 6:97–102.
Article26. Bendapudi PK, Hurwitz S, Fry A, et al. Derivation and external validation of the PLASMIC score for rapid assessment of adults with thrombotic microangiopathies: a cohort study. Lancet Haematol. 2017; 4:e157–e164.
Article27. Lee JM, Park YS, Lee JH, et al. Atypical hemolytic uremic syndrome: Korean pediatric series. Pediatr Int. 2015; 57:431–438.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Case Report of Pregnancy Induced Atypical Hemolytic-uremic Syndrome
- Atypical hemolytic uremic syndrome and eculizumab therapy in children
- Atypical Hemolytic Uremic Syndrome in a 13-year-old Lao Girl: A Case Report
- Recurrent hemolytic uremic syndrome caused by DGKE gene mutation: a case report
- A new pathological perspective on thrombotic microangiopathy