Ann Clin Microbiol.
2019 Sep;22(3):61-70. 10.5145/ACM.2019.22.3.61.
Performance Evaluation of Newly Developed Korean Antimicrobial Susceptibility Testing Panels for MicroScan System Using Clinical Isolates from Teaching Hospitals in Korea
- Affiliations
-
- 1Department of Laboratory Medicine and Research Institute of Bacterial Resistance, Yonsei University College of Medicine, Seoul, Korea. kyunsky@yuhs.ac
- 2Department of Clinical Pathology, Sangji University College of Health Science, Wonju, Korea.
- KMID: 2459177
- DOI: http://doi.org/10.5145/ACM.2019.22.3.61
Abstract
- BACKGROUND
Antimicrobial resistant continues to pose a threat to public health. Therefore, rapid and accurate antimicrobial susceptibility testing is very important. The objectives of this study were to evaluate the performance of the MicroScan system (Beckman Coulter, USA) with newly developed Korean Antimicrobial Susceptibility Testing Panels (KSCM panels) for antimicrobial susceptibility testing (AST) against clinical isolates in South Korea.
METHODS
Three KSCM panels were designed in this study. For the performance evaluation, a total of 1,325 clinical isolates including 1,027 of Gram-negative bacilli and 298 Gram-positive cocci collected from eight general hospitals in South Korea were used. The results by KSCM panels were compared with those by conventional methods.
RESULTS
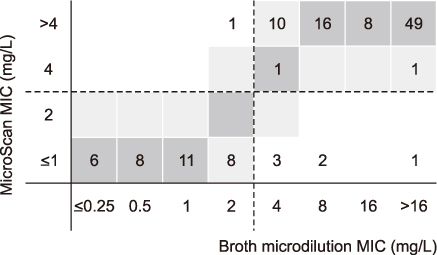
By KSCM-1 panel for Gram-positive cocci, the rates of categorical agreement (CA) were >90% in all the antimicrobials tested in this study. The rates of major error (ME) were also <3%, and only three very major error (VME) were identified; each of ampicillin, tetracycline, and quinupristin-dalfopristin in enterococcal isolates. By KSCM-2 panel for Enterobacteriaceae, the rates of CA were also above 90%, and those of ME and VME were less than 3% and 1.5%, respectively. KSCM-3 panels for glucose- non-fermenting Gram-negative bacilli, also showed good agreement rates, i.e., CA rates >90%, ME rates <3%, and VME rates <1.5%.
CONCLUSION
The newly developed three KSCM panels for MicroScan system (Beckman Coulter) showed excellent performance in AST against a large number of clinical isolates, and they are applicable to clinical microbiology laboratories.
MeSH Terms
Figure
Reference
-
1. Stefani S, Chung DR, Lindsay JA, Friedrich AW, Kearns AM, Westh H, et al. Meticillin-resistant Staphylococcus aureus (MRSA): global epidemiology and harmonisation of typing methods. Int J Antimicrob Agents. 2012; 39:273–282.2. Cheong HJ, Song JY, Eom JS, Kim WJ, Choi SJ, Choi JH, et al. Colonization rate, risk factor for acquisition and genetic diversity of vancomycin-resistant enterococci (VRE) isolated from rectal culture of patients in intensive care units from ten large hospitals in Korea. Korean J Infect Dis. 2002; 34:276–284.3. Kim B, Kim J, Seo MR, Wie SH, Cho YK, Lim SK, et al. Clinical characteristics of community-acquired acute pyelonephritis caused by ESBL-producing pathogens in South Korea. Infection. 2013; 41:603–612.
Article4. Gupta N, Limbago BM, Patel JB, Kallen AJ. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis. 2011; 53:60–67.
Article5. Lee JY, Ko KS. OprD mutations and inactivation, expression of efflux pumps and AmpC, and metallo-β-lactamases in carbapenem-resistant Pseudomonas aeruginosa isolates from South Korea. Int J Antimicrob Agents. 2012; 40:168–172.6. Perez F, Hujer AM, Hujer KM, Decker BK, Rather PN, Bonomo RA. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2007; 51:3471–3484.7. Kim D, Ahn JY, Lee CH, Jang SJ, Lee H, Yong D, et al. Increasing resistance to extended-spectrum cephalosporins, fluoroquinolone, and carbapenem in gram-negative bacilli and the emergence of carbapenem non-susceptibility in Klebsiella pneumoniae: analysis of Korean Antimicrobial Resistance Monitoring System (KARMS) data from 2013 to 2015. Ann Lab Med. 2017; 37:231–239.8. Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012; 18:268–281.
Article9. CLSI. Performance standards for antimicrobial susceptibility testing. CLSI document M100. Wayne, PA: Clinical and Laboratory Standards Institute;2018.10. European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 8.0. 31 January 2018. http://www.eucast.org/ast_of_bacteria/previous_versions_of_documents/[Online].11. Yoon YK, Kim JY, Park DW, Sohn JW, Kim MJ. Predictors of persistent methicillin-resistant Staphylococcus aureus bacteraemia in patients treated with vancomycin. J Antimicrob Chemother. 2010; 65:1015–1018.12. Choi HG, Park KH, Park SN, Jun BC, Lee DH, Yeo SW. The appropriate medical management of methicillin-resistant Staphylococcus aureus in chronic suppurative otitis media. Acta Otolaryngol. 2010; 130:42–46.13. Steers E, Foltz EL, Graves BS. An inocula replicating apparatus for routine testing of bacterial susceptibility to antibiotics. Antibiot Chemother (Northfield). 1959; 9:307–311.14. European Committee on Antimicrobial Susceptibility Testing. Recommendations for MIC determination of colistin (polymyxin E) as recommended by the joint CLSI-EUCAST Polymyxin Breakpoints Working Group. 31 March 2016. http://www.eucast.org/ast_of_bacteria/guidance_documents/[Online].15. CLSI. Verification of commercial microbial identification and antimicrobial susceptibility testing systems. CLSI document M52. Wayne, PA: Clinical and Laboratory Standards Institute;2015.16. Levy SB. Antibiotic and antiseptic resistance: impact on public health. Pediatr Infect Dis J. 2000; 19(10 Suppl):S120–S122.
Article17. Felten A, Grandry B, Lagrange PH, Casin I. Evaluation of three techniques for detection of low-level methicillin-resistant Staphylococcus aureus (MRSA): a disk diffusion method with cefoxitin and moxalactam, the Vitek 2 system, and the MRSA-screen latex agglutination test. J Clin Microbiol. 2002; 40:2766–2771.18. Fernandes CJ, Fernandes LA, Collignon P. Australian Group on Antimicrobial Resistance. Cefoxitin resistance as a surrogate marker for the detection of methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 2005; 55:506–510.19. Villegas-Estrada A, Lee M, Hesek D, Vakulenko SB, Mobashery S. Co-opting the cell wall in fighting methicillin-resistant Staphylococcus aureus: potent inhibition of PBP 2a by two anti-MRSA beta-lactam antibiotics. J Am Chem Soc. 2008; 130:9212–9213.20. Lee H, Yoon EJ, Kim D, Kim JW, Lee KJ, Kim HS, et al. Ceftaroline resistance by clone-specific polymorphism in penicillinbinding protein 2a of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2018; 62:e00485-18.
Article21. Lo-Ten-Foe JR, de Smet AM, Diederen BM, Kluytmans JA, van Keulen PH. Comparative evaluation of the VITEK 2, disk diffusion, Etest, broth microdilution, and agar dilution susceptibility testing methods for colistin in clinical isolates, including heteroresistant Enterobacter cloacae and Acinetobacter baumannii strains. Antimicrob Agents Chemother. 2007; 51:3726–3730.22. Malaviolle X, Nonhoff C, Denis O, Rottiers S, Struelens MJ. Evaluation of disc diffusion methods and Vitek 2 automated system for testing susceptibility to mupirocin in Staphylococcus aureus. J Antimicrob Chemother. 2008; 62:1018–1023.23. Donay JL, Mathieu D, Fernandes P, Prégermain C, Bruel P, Wargnier A, et al. Evaluation of the automated Phoenix system for potential routine use in the clinical microbiology laboratory. J Clin Microbiol. 2004; 42:1542–1546.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of identification in Enterococcus Species by Using MicroScan(R)Panels
- Comparison of the MicroScan(R) Combo Panel Synergies plus with the MicroScan(R) Conventional Combo Panel for Diagnostic Performance of Gram-negative and Gram-positive Bacteria
- Evaluation of MicroScan MICroSTREP Plus Antimicrobial Susceptibility Panel for Testing Streptococcus pneumoniae
- Evaluation of MicroScan Synergies plus Positive Combo 3 Panels for Identification and Antimicrobial Susceptibility Testing of Staphylococcus aureus and Enterococcus Species
- Discordance in Colistin Susceptibility Test for Acinetobacter baumannii Showing Resistance: MicroScan versus Etest