Ann Dermatol.
2018 Aug;30(4):451-453. 10.5021/ad.2018.30.4.451.
Olmutinib Induced Lichen Planus Like Eruption
- Affiliations
-
- 1Department of Dermatology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. jh1204.park@samsung.com
- KMID: 2457522
- DOI: http://doi.org/10.5021/ad.2018.30.4.451
Abstract
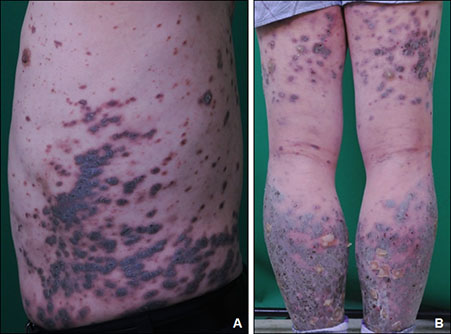
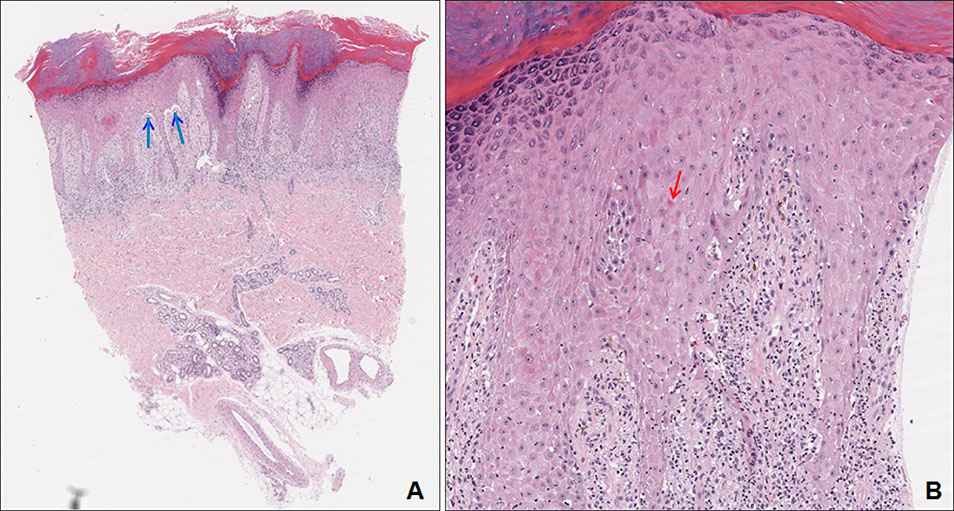
- Drug induced lichen planus like eruption is an uncommon cutaneous adverse effect of several drugs. This appears symmetric eruption of erythematous or violaceous plaques resembling lichen planus on the trunk and extremities. A 50-year-old male presented with scaly, violaceous plaques and dusky brown macules on whole body. For four months, the patient was treated with olmutinib, an oral, third-generation epidermal growth factor receptor-tyrosine kinase inhibitor. In May 2016, olmutinib received its first global approval in South Korea for the treatment of patients with locally advanced or metastatic epidermal growth factor receptor T790M mutation-positive non-small cell lung cancer. The biopsy specimen from the patient showed features of lichen planus. We diagnosed him with olmutinib-induced lichen planus like eruption. He was treated with oral methylprednisolone and topical desoxymethasone 0.25% ointment. At the same time, olmutinib dose was decreased to three-fourths of this patient's starting dose. After that, the cutaneous lesions improved.
MeSH Terms
-
Biopsy
Carcinoma, Non-Small-Cell Lung
Desoximetasone
Drug Eruptions
Epidermal Growth Factor
Extremities
Humans
Korea
Lichen Planus*
Lichens*
Male
Methylprednisolone
Middle Aged
Phosphotransferases
Receptor, Epidermal Growth Factor
Desoximetasone
Epidermal Growth Factor
Methylprednisolone
Phosphotransferases
Receptor, Epidermal Growth Factor
Figure
Reference
-
1. Kim ES. Olmutinib: first global approval. Drugs. 2016; 76:1153–1157.
Article2. Ellgehausen P, Elsner P, Burg G. Drug-induced lichen planus. Clin Dermatol. 1998; 16:325–332.
Article3. Brauer J, Votava HJ, Meehan S, Soter NA. Lichenoid drug eruption. Dermatol Online J. 2009; 15:13.
Article4. Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, et al. Fitzpatrick's dermatology in general medicine. 8th ed. New York: McGraw-Hill Medical;2012. p. 304.5. Agero AL, Dusza SW, Benvenuto-Andrade C, Busam KJ, Myskowski P, Halpern AC. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol. 2006; 55:657–670.
Article6. Lacouture ME, Schadendorf D, Chu CY, Uttenreuther-Fischer M, Stammberger U, O'Brien D, et al. Dermatologic adverse events associated with afatinib: an oral ErbB family blocker. Expert Rev Anticancer Ther. 2013; 13:721–728.
Article7. Patel AB, Solomon AR, Mauro MJ, Ehst BD. Unique cutaneous reaction to second- and third-generation tyrosine kinase inhibitors for chronic myeloid leukemia. Dermatology. 2016; 232:122–125.
Article8. Pretel-Irazabal M, Tuneu-Valls A, Ormaechea-Perez N. [Adverse skin effects of imatinib, a tyrosine kinase inhibitor]. Actas Dermosifiliogr. 2014; 105:655–662. Spanish.
Article9. Liao BC, Lin CC, Lee JH, Yang JC. Update on recent preclinical and clinical studies of T790M mutant-specific irreversible epidermal growth factor receptor tyrosine kinase inhibitors. J Biomed Sci. 2016; 23:86.
Article10. Lee KO, Cha MY, Kim M, Song JY, Lee JH, Kim YH, et al. Abstract LB-100: Discovery of HM61713 as an orally available and mutant EGFR selective inhibitor. Cancer Res. 2014; 74:19 Suppl. LB-100.
Article