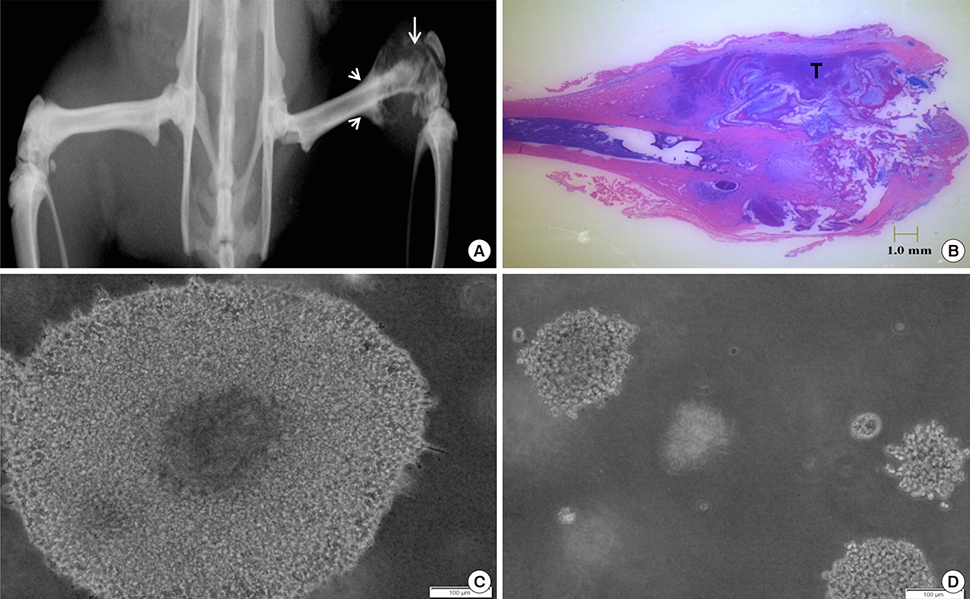
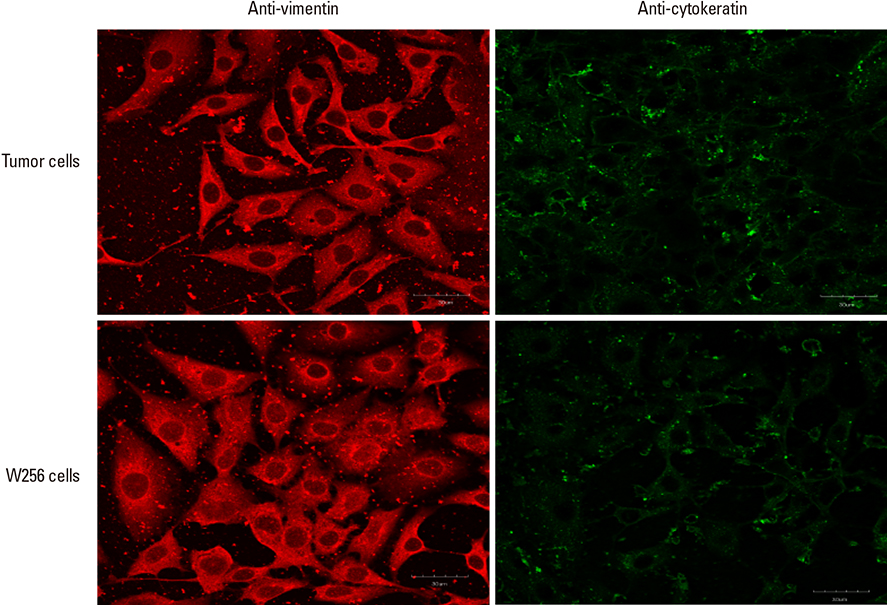
J Bone Metab.
2019 Aug;26(3):179-191. 10.11005/jbm.2019.26.3.179.
Mechanical and Mechanosensing Properties of Tumor Affected Bone Cells Were Inhibited via PI3K/Akt Pathway
- Affiliations
-
- 1Division of Mechanical and Biomedical Engineering, Ewha Womans University, Seoul, Korea. tlee@ewha.ac.kr
- KMID: 2457437
- DOI: http://doi.org/10.11005/jbm.2019.26.3.179
Abstract
- BACKGROUND
Osteolytic metastasis is a common destructive form of metastasis, in which there is an increased bone resorption but impaired bone formation. It is hypothesized that the changed mechanical properties of tumor affected bone cells could inhibit its mechanosensing, thus contributing to differences in bone remodeling.
METHODS
Here, atomic force microscopy indentation on primary bone cells exposed to 50% conditioned medium from Walker 256 (W) carcinoma cell line or its adaptive tumor (T) cells was carried out. Nitric oxide levels of bone cells were monitored in response to low-magnitude, high-frequency (LMHF) vibrations.
RESULTS
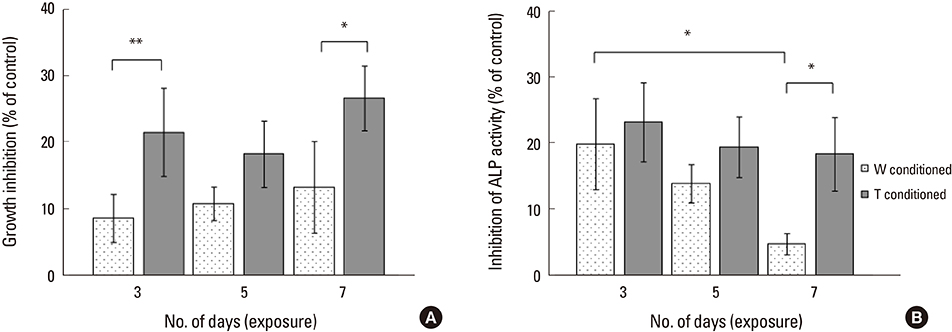
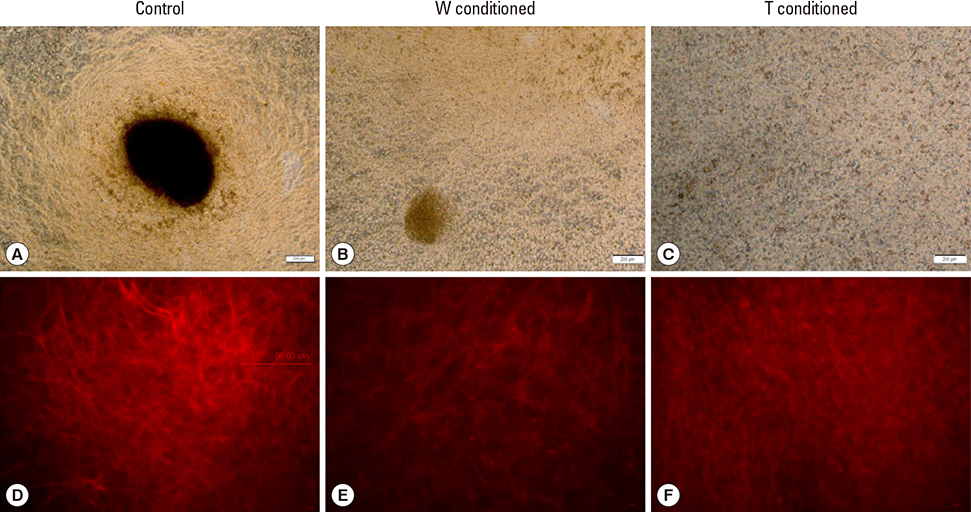
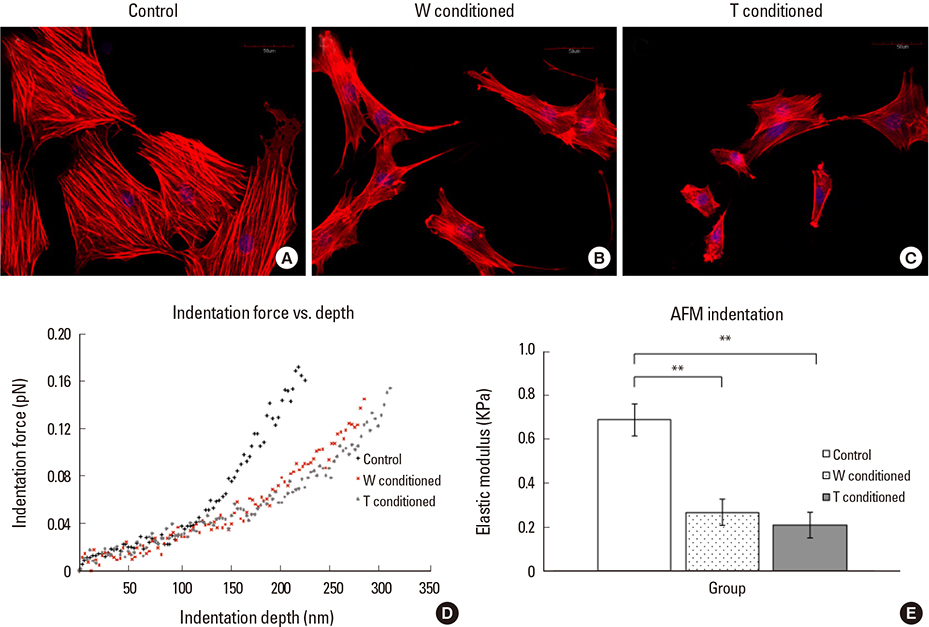
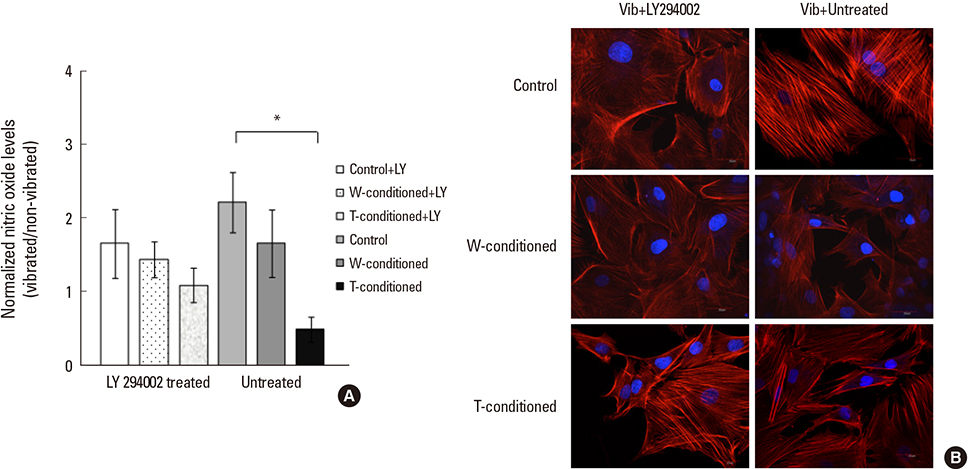
A stronger sustained inhibitive effect on bone cell viability and differentiation by T cells as compared to that of its cell line was demonstrated. This could be attributed to the higher levels of transforming growth factor-β1 (TGF-β1) in the T-conditioned medium as compared to W-conditioned medium. Bone cell elastic moduli in W and T-groups were found to decrease significantly by 61.0% and 69.6%, respectively compared to control and corresponded to filamentous actin changes. Nitric oxide responses were significantly inhibited in T-conditioned group but not in W-conditioned group.
CONCLUSIONS
It implied that a change in cell mechanical properties is not sufficient as an indicator of change in mechanosensing ability. Moreover, inhibition of phosphoinositide 3-kinase/Akt downstream signaling pathway of TGF-β1 alleviated the inhibition effects on mechanosensing in T-conditioned cells, further suggesting that growth factors such as TGF-β could be good therapeutic targets for osteoblast treatment.
MeSH Terms
-
Actins
Bone Neoplasms
Bone Remodeling
Bone Resorption
Cell Line
Cell Survival
Culture Media, Conditioned
Elastic Modulus
Intercellular Signaling Peptides and Proteins
Microscopy, Atomic Force
Neoplasm Metastasis
Nitric Oxide
Osteoblasts
Osteogenesis
Sensitivity Training Groups
T-Lymphocytes
Vibration
Walkers
Actins
Culture Media, Conditioned
Intercellular Signaling Peptides and Proteins
Nitric Oxide
Figure
Reference
-
1. Bussard KM, Mastro AM. Ex-vivo analysis of the bone microenvironment in bone metastatic breast cancer. J Mammary Gland Biol Neoplasia. 2009; 14:387–395.
Article2. Kakonen SM, Mundy GR. Mechanisms of osteolytic bone metastases in breast carcinoma. Cancer. 2003; 97:834–839.
Article3. Kozlow W, Guise TA. Breast cancer metastasis to bone: mechanisms of osteolysis and implications for therapy. J Mammary Gland Biol Neoplasia. 2005; 10:169–180.
Article4. Phadke PA, Mercer RR, Harms JF, et al. Kinetics of metastatic breast cancer cell trafficking in bone. Clin Cancer Res. 2006; 12:1431–1440.
Article5. Sasaki A, Boyce BF, Story B, et al. Bisphosphonate risedronate reduces metastatic human breast cancer burden in bone in nude mice. Cancer Res. 1995; 55:3551–3557.6. Mastro AM, Gay CV, Welch DR, et al. Breast cancer cells induce osteoblast apoptosis: a possible contributor to bone degradation. J Cell Biochem. 2004; 91:265–276.
Article7. Mercer RR, Miyasaka C, Mastro AM. Metastatic breast cancer cells suppress osteoblast adhesion and differentiation. Clin Exp Metastasis. 2004; 21:427–435.
Article8. Zhu GY, Zhang YY, Gu SZ, et al. Inhibitory effects of breast cancer cells on proliferation and differentiation of osteoblasts. Ai Zheng. 2009; 28:449–455.9. Mercer RR, Mastro AM. Cytokines secreted by bone-metastatic breast cancer cells alter the expression pattern of f-actin and reduce focal adhesion plaques in osteoblasts through PI3K. Exp Cell Res. 2005; 310:270–281.
Article10. Adachi T, Sato K, Higashi N, et al. Simultaneous observation of calcium signaling response and membrane deformation due to localized mechanical stimulus in single osteoblast-like cells. J Mech Behav Biomed Mater. 2008; 1:43–50.
Article11. Kreja L, Liedert A, Hasni S, et al. Mechanical regulation of osteoclastic genes in human osteoblasts. Biochem Biophys Res Commun. 2008; 368:582–587.
Article12. Tanaka SM, Sun HB, Roeder RK, et al. Osteoblast responses one hour after load-induced fluid flow in a three-dimensional porous matrix. Calcif Tissue Int. 2005; 76:261–271.
Article13. Li J, Chen G, Zheng L, et al. Osteoblast cytoskeletal modulation in response to compressive stress at physiological levels. Mol Cell Biochem. 2007; 304:45–52.
Article14. Bacabac RG, Mizuno D, Schmidt CF, et al. Round versus flat: bone cell morphology, elasticity, and mechanosensing. J Biomech. 2008; 41:1590–1598.
Article15. Sugawara Y, Ando R, Kamioka H, et al. The alteration of a mechanical property of bone cells during the process of changing from osteoblasts to osteocytes. Bone. 2008; 43:19–24.
Article16. Zhou ZL, Ngan AH, Tang B, et al. Reliable measurement of elastic modulus of cells by nanoindentation in an atomic force microscope. J Mech Behav Biomed Mater. 2012; 8:134–142.
Article17. Lim CT, Zhou EH, Li A, et al. Experimental techniques for single cell and single molecule biomechanics. Mater Sci Eng C. 2006; 26:1278–1288.
Article18. Bacabac RG, Smit TH, Van Loon JJ, et al. Bone cell responses to high-frequency vibration stress: does the nucleus oscillate within the cytoplasm. Faseb J. 2006; 20:858–864.
Article19. Hou WW, Zhu ZL, Zhou Y, et al. Involvement of Wnt activation in the micromechanical vibration-enhanced osteogenic response of osteoblasts. J Orthop Sci. 2011; 16:598–605.
Article20. Patel MJ, Chang KH, Sykes MC, et al. Low magnitude and high frequency mechanical loading prevents decreased bone formation responses of 2T3 preosteoblasts. J Cell Biochem. 2009; 106:306–316.
Article21. Prè D, Ceccarelli G, Gastaldi G, et al. The differentiation of human adipose-derived stem cells (hASCs) into osteoblasts is promoted by low amplitude, high frequency vibration treatment. Bone. 2011; 49:295–303.
Article22. Shi HF, Cheung WH, Qin L, et al. Low-magnitude high-frequency vibration treatment augments fracture healing in ovariectomy-induced osteoporotic bone. Bone. 2010; 46:1299–1305.
Article23. Turner CH, Takano Y, Owan I, et al. Nitric oxide inhibitor L-NAME suppresses mechanically induced bone formation in rats. Am J Physiol. 1996; 270:E634–E639.
Article24. Chen X, Goh JC, Teoh SH, et al. Localized sclerotic bone response demonstrated reduced nanomechanical creep properties. J Mech Behav Biomed Mater. 2013; 17:198–208.
Article25. Masters JRW, editor. Human cancer in primary culture, a handbook. Dordrecht, NL: Kluwer Academic Publishers;1991.26. Li L, Price JE, Fan D, et al. Correlation of growth capacity of human tumor cells in hard agarose with their in vivo proliferative capacity at specific metastatic sites. J Natl Cancer Inst. 1989; 81:1406–1412.
Article27. Jaasma MJ, Jackson WM, Keaveny TM. The effects of morphology, confluency, and phenotype on whole-cell mechanical behavior. Ann Biomed Eng. 2006; 34:759–768.
Article28. Muthukumaran P, Lim CT, Lee T. Estradiol influences the mechanical properties of human fetal osteoblasts through cytoskeletal changes. Biochem Biophys Res Commun. 2012; 423:503–508.
Article29. Darling EM, Topel M, Zauscher S, et al. Viscoelastic properties of human mesenchymally-derived stem cells and primary osteoblasts, chondrocytes, and adipocytes. J Biomech. 2008; 41:454–464.
Article30. Li QS, Lee GY, Ong CN, et al. AFM indentation study of breast cancer cells. Biochem Biophys Res Commun. 2008; 374:609–613.
Article31. Bullough PG. Orthopaedic pathology. 5th ed. Maryland Heights, MO: Mosby;2010.32. Simpkins H, Lehman JM, Mazurkiewicz JE, et al. A morphological and phenotypic analysis of Walker 256 cells. Cancer Res. 1991; 51:1334–1338.33. Dallas SL, Park-Snyder S, Miyazono K, et al. Characterization and autoregulation of latent transforming growth factor beta (TGF beta) complexes in osteoblast-like cell lines. Production of a latent complex lacking the latent TGF beta-binding protein. J Biol Chem. 1994; 269:6815–6821.
Article34. Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003; 3:453–458.
Article35. Li RD, Deng ZL, Hu N, et al. Biphasic effects of TGFbeta1 on BMP9-induced osteogenic differentiation of mesenchymal stem cells. BMB Rep. 2012; 45:509–514.
Article36. Zhao L, Jiang S, Hantash BM. Transforming growth factor beta1 induces osteogenic differentiation of murine bone marrow stromal cells. Tissue Eng Part A. 2010; 16:725–733.
Article37. Zhou S. TGF-beta regulates beta-catenin signaling and osteoblast differentiation in human mesenchymal stem cells. J Cell Biochem. 2011; 112:1651–1660.
Article38. Dufour C, Holy X, Marie PJ. Transforming growth factor-beta prevents osteoblast apoptosis induced by skeletal unloading via PI3K/Akt, Bcl-2, and phospho-Bad signaling. Am J Physiol Endocrinol Metab. 2008; 294:E794–E801.39. Ochiai H, Okada S, Saito A, et al. Inhibition of insulin-like growth factor-1 (IGF-1) expression by prolonged transforming growth factor-beta1 (TGF-beta1) administration suppresses osteoblast differentiation. J Biol Chem. 2012; 287:22654–22661.
Article40. Dhurjati R, Krishnan V, Shuman LA, et al. Metastatic breast cancer cells colonize and degrade three-dimensional osteoblastic tissue in vitro. Clin Exp Metastasis. 2008; 25:741–752.
Article41. Lynch MP, Stein JL, Stein GS, et al. The influence of type I collagen on the development and maintenance of the osteoblast phenotype in primary and passaged rat calvarial osteoblasts: modification of expression of genes supporting cell growth, adhesion, and extracellular matrix mineralization. Exp Cell Res. 1995; 216:35–45.
Article42. Rotsch C, Radmacher M. Drug-induced changes of cytoskeletal structure and mechanics in fibroblasts: an atomic force microscopy study. Biophys J. 2000; 78:520–535.
Article43. Takai E, Costa KD, Shaheen A, et al. Osteoblast elastic modulus measured by atomic force microscopy is substrate dependent. Ann Biomed Eng. 2005; 33:963–971.
Article44. Myers KA, Rattner JB, Shrive NG, et al. Osteoblast-like cells and fluid flow: cytoskeleton-dependent shear sensitivity. Biochem Biophys Res Commun. 2007; 364:214–219.
Article45. Mikuni-Takagaki Y, Suzuki Y, Kawase T, et al. Distinct responses of different populations of bone cells to mechanical stress. Endocrinology. 1996; 137:2028–2035.
Article46. Vlahos CJ, Matter WF, Hui KY, et al. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J Biol Chem. 1994; 269:5241–5248.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- CDK1 promotes the phosphorylation of KIFC1 to regulate the tumorgenicity of endometrial carcinoma
- Vitexin Inhibits Gastric Cancer Growth and Metastasis through HMGB1-mediated Inactivation of the PI3K/ AKT/mTOR/HIF-1α Signaling Pathway
- Developmental Exposure to Di-(2-ethylhexyl) Phthalate Induces Cerebellar Granule Cell Apoptosis via the PI3K/AKT Signaling Pathway
- Isorhamnetin Alleviates Inflammation-Induced Crosstalk between Kynurenine Pathway and Gut Microbiota in Depressed Mice
- MSCs-Derived miR-150-5p-Expressing Exosomes Promote Skin Wound Healing by Activating PI3K/AKT Pathway through PTEN