J Bone Metab.
2019 Aug;26(3):169-177. 10.11005/jbm.2019.26.3.169.
Effects of Di(2-ethylhexyl)phthalate on Bone Metabolism in Ovariectomized Mice
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Soonchunhyang University College of Medicine, Bucheon, Korea.
- 2Department of Obstetrics and Gynecology, Eunpyeong St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. drrabbit@catholic.ac.kr
- KMID: 2457436
- DOI: http://doi.org/10.11005/jbm.2019.26.3.169
Abstract
- BACKGROUND
The molecular pathways of how endocrine disruptors affect bone mineral density (BMD) and bone remodeling are still unclear. The purpose of this experimental study is to determine the effects of di(2-ethylhexyl)phthalate (DEHP) on bone metabolism in ovariectomized mice.
METHODS
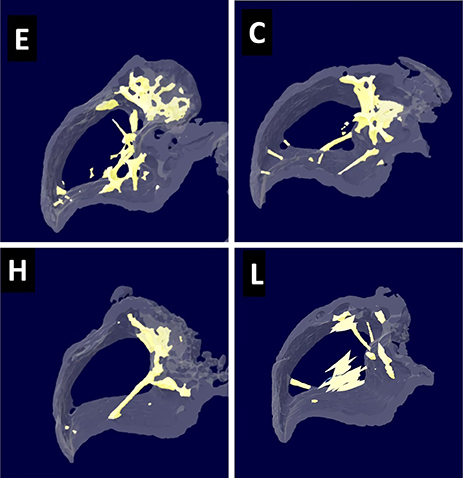
Twenty-six-month-old female CD-1 mice were divided into 4 groups: control, low-dose DEHP, high-dose DEHP, and estrogen groups (n=5, each group). All mice were subjected to ovariectomy for the induction of artificial menopause and then exposed to corn oil, DEHP, and estrogen for 2 months. Micro-computed tomography (Micro-CT) of the bone and analysis of blood samples for bone markers were performed to observe the changes in bone metabolism.
RESULTS
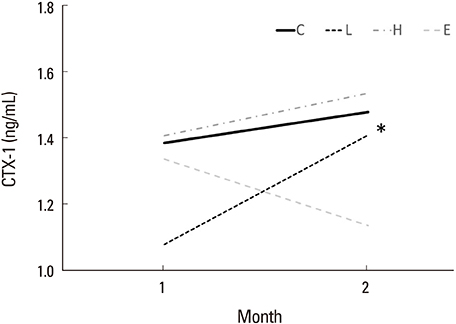
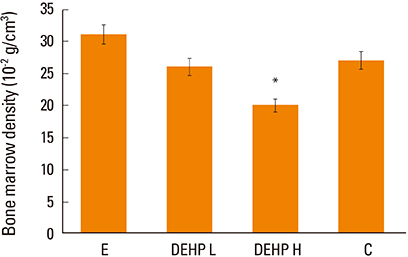
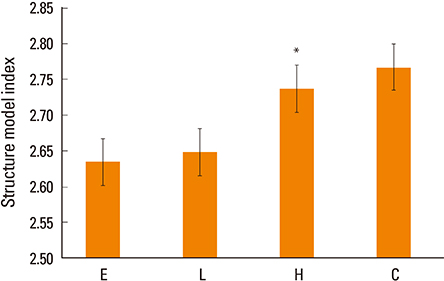
Osteocalcin level was decreased in the control, low-dose and high-dose DEHP group, the reduction width was greater in the high-dose DEHP group (−0.219 ng/mL) than control group (−0.077 ng/mL, P<0.05). C-terminal telopeptide of type I collagen level was increased in the control, low-dose and high-dose DEHP group, the increase range of low-dose DEHP group (0.329 ng/mL) showed greater than control group (0.093 ng/mL, P<0.05). Micro-CT analysis revealed that the BMD was significantly lower in the high-dose DEHP group (19.8×10⻲ g/cm³) than control group (27.2×10⻲ g/cm³, P<0.05). The structure model index was significantly higher in the high-dose DEHP group (2.737) than low-dose DEHP group (2.648) and estrogen group (2.63, P<0.05). It means the progression of osteoporosis in the high-dose DEHP group.
CONCLUSIONS
These results confirm the negative effects of DEHP on bone health in ovariectomized mice. Further continuous studies on genetic pathways and other endocrine disruptors will be necessary to validate these findings.
MeSH Terms
Figure
Cited by 1 articles
-
Effects of Endocrine-Disrupting Chemicals on Bone Health
So Young Park, Sung Hye Kong, Kyoung Jin Kim, Seong Hee Ahn, Namki Hong, Jeonghoon Ha, Sihoon Lee, Han Seok Choi, Ki-Hyun Baek, Jung-Eun Kim, Sang Wan Kim
Endocrinol Metab. 2024;39(4):539-551. doi: 10.3803/EnM.2024.1963.
Reference
-
1. Roy JR, Chakraborty S, Chakraborty TR. Estrogen-like endocrine disrupting chemicals affecting puberty in humans--a review. Med Sci Monit. 2009; 15:Ra137–Ra145.2. Migliaccio S, Newbold RR, Bullock BC, et al. Alterations of maternal estrogen levels during gestation affect the skeleton of female offspring. Endocrinology. 1996; 137:2118–2125.
Article3. Migliaccio S, Newbold RR, Teti A, et al. Transient estrogen exposure of female mice during early development permanently affects osteoclastogenesis in adulthood. Bone. 2000; 27:47–52.
Article4. Hermsen SA, Larsson S, Arima A, et al. In utero and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) affects bone tissue in rhesus monkeys. Toxicology. 2008; 253:147–152.
Article5. Sabbieti MG, Agas D, Santoni G, et al. Involvement of p53 in phthalate effects on mouse and rat osteoblasts. J Cell Biochem. 2009; 107:316–327.
Article6. Agas D, Sabbieti MG, Marchetti L. Endocrine disruptors and bone metabolism. Arch Toxicol. 2013; 87:735–751.
Article7. Harris CA, Henttu P, Parker MG, et al. The estrogenic activity of phthalate esters in vitro. Environ Health Perspect. 1997; 105:802–811.
Article8. Okubo T, Suzuki T, Yokoyama Y, et al. Estimation of estrogenic and anti-estrogenic activities of some phthalate diesters and monoesters by MCF-7 cell proliferation assay in vitro. Biol Pharm Bull. 2003; 26:1219–1224.
Article9. Hurst CH, Waxman DJ. Activation of PPARalpha and PPAR-gamma by environmental phthalate monoesters. Toxicol Sci. 2003; 74:297–308.10. Cheon KY, Kil KH, Choi JI, et al. Parenteral exposure to DEHP and its effect on the microstructure of bone and Wnt signaling pathway in F2 female mice. Biochip J. 2016; 10:233–240.
Article11. Min KB, Min JY. Urinary phthalate metabolites and the risk of low bone mineral density and osteoporosis in older women. J Clin Endocrinol Metab. 2014; 99:E1997–E2003.
Article12. Koo HJ, Lee BM. Estimated exposure to phthalates in cosmetics and risk assessment. J Toxicol Environ Health A. 2004; 67:1901–1914.
Article13. Guo Y, Wang L, Kannan K. Phthalates and parabens in personal care products from China: concentrations and human exposure. Arch Environ Contam Toxicol. 2014; 66:113–119.
Article14. Luo H, Sun G, Shi Y, et al. Evaluation of the Di(2-ethylhexyl)phthalate released from polyvinyl chloride medical devices that contact blood. Springerplus. 2014; 3:58.
Article15. Huygh J, Clotman K, Malarvannan G, et al. Considerable exposure to the endocrine disrupting chemicals phthalates and bisphenol-A in intensive care unit (ICU) patients. Environ Int. 2015; 81:64–72.
Article16. Gordon SR. Microfilament disruption in a noncycling organized tissue, the corneal endothelium, initiates mitosis. Exp Cell Res. 2002; 272:127–134.
Article17. Agarwal DK, Maronpot RR, Lamb JCt, et al. Adverse effects of butyl benzyl phthalate on the reproductive and hematopoietic systems of male rats. Toxicology. 1985; 35:189–206.
Article18. Bhat FA, Ramajayam G, Parameswari S, et al. Di 2-ethyl hexyl phthalate affects differentiation and matrix mineralization of rat calvarial osteoblasts--in vitro. Toxicol In Vitro. 2013; 27:250–256.
Article19. Akune T, Ohba S, Kamekura S, et al. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004; 113:846–855.
Article20. Grey A, Bolland M, Gamble G, et al. The peroxisome proliferator-activated receptor-gamma agonist rosiglitazone decreases bone formation and bone mineral density in healthy postmenopausal women: a randomized, controlled trial. J Clin Endocrinol Metab. 2007; 92:1305–1310.
Article21. Lecomte S, Demay F, Ferrière F, et al. Phytochemicals targeting estrogen receptors: Beneficial rather than adverse effects? Int J Mol Sci. 2017; 18:E1381.
Article22. Rusyn I, Peters JM, Cunningham ML. Modes of action and species-specific effects of di-(2-ethylhexyl)phthalate in the liver. Crit Rev Toxicol. 2006; 36:459–479.
Article23. Hannon PR, Brannick KE, Wang W, et al. Di(2-ethylhexyl) phthalate inhibits antral follicle growth, induces atresia, and inhibits steroid hormone production in cultured mouse antral follicles. Toxicol Appl Pharmacol. 2015; 284:42–53.
Article24. Hannon PR, Peretz J, Flaws JA. Daily exposure to Di(2-ethylhexyl) phthalate alters estrous cyclicity and accelerates primordial follicle recruitment potentially via dysregulation of the phosphatidylinositol 3-kinase signaling pathway in adult mice. Biol Reprod. 2014; 90:136.
Article25. Chiu CY, Sun SC, Chiang CK, et al. Plasticizer di(2-ethylhexyl) phthalate interferes with osteoblastogenesis and adipogenesis in a mouse model. J Orthop Res. 2018; 36:1124–1134.26. DeFlorio-Barker SA, Turyk ME. Associations between bone mineral density and urinary phthalate metabolites among post-menopausal women: a cross-sectional study of NHANES data 2005-2010. Int J Environ Health Res. 2016; 26:326–345.
Article27. Agency for Toxic Substances and Disease Registry. Toxicological profile for di(2-Ethylhexyl)phthalate (DEHP). 2002. cited by 2017 Sep 1. Available from: https://www.atsdr.cdc.gov/ToxProfiles/tp.asp?id=684&tid=65.28. Cho HH, Kim GW, Ryu JC. The effects of Di-2-ethylhexyl phthalates (DEHP) on the cell cycle of the endometrial cancer cell lines (ECC-1). Toxicol Environ Health Sci. 2014; 6:217–223.
Article29. Khadka B, Tiwari ML, Gautam R, et al. Correlates of biochemical markers of bone turnover among post-menopausal women. JNMA J Nepal Med Assoc. 2018; 56:754–758.
Article30. Hildebrand T, Rüegsegger P. Quantification of bone microarchitecture with the structure model index. Comput Methods Biomech Biomed Engin. 1997; 1:15–23.
Article31. Grote HJ, Amling M, Vogel M, et al. Intervertebral variation in trabecular microarchitecture throughout the normal spine in relation to age. Bone. 1995; 16:301–308.
Article32. Ding M, Hvid I. Quantification of age-related changes in the structure model type and trabecular thickness of human tibial cancellous bone. Bone. 2000; 26:291–295.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Age-dependent Effect of Metabolism and Testicular Toxicity to di(2-ethylhexyl) Phthalate
- Determination of Phthalate Metabolites in Human Serum and Urine as Biomarkers for Phthalate Exposure Using Column-Switching LC-MS/MS
- Antimicrobial and Cytotoxic Activity of Di-(2-ethylhexyl) Phthalate and Anhydrosophoradiol-3-acetate Isolated from Calotropis gigantea (Linn.) Flower
- Developmental Exposure to Di-(2-ethylhexyl) Phthalate Induces Cerebellar Granule Cell Apoptosis via the PI3K/AKT Signaling Pathway
- Short-term exposure to di(2-ethylhexyl)phthalate may disrupt hepatic lipid metabolism through modulating the oxidative stress in male adolescent rats