Ann Dermatol.
2019 Oct;31(5):538-544. 10.5021/ad.2019.31.5.538.
Therapeutic Effect of Glucosamine on an Atopic Dermatitis Animal Model
- Affiliations
-
- 1Department of Dermatology, Inha University School of Medicine, Incheon, Korea. garden@inha.ac.kr
- 2Department of Otorhinolaryngology-Head and Neck Surgery, Inha University School of Medicine, Incheon, Korea.
- KMID: 2456231
- DOI: http://doi.org/10.5021/ad.2019.31.5.538
Abstract
- BACKGROUND
Recent studies have reported that glucosamine (GlcN) showed therapeutic effects in allergic diseases such as asthma and rhinitis, and its mechanisms include the suppression of T helper type 2 immune responses and the nuclear factor-κB pathway.
OBJECTIVE
We aimed to investigate the effect of GlcN on atopic dermatitis (AD) in an animal model.
METHODS
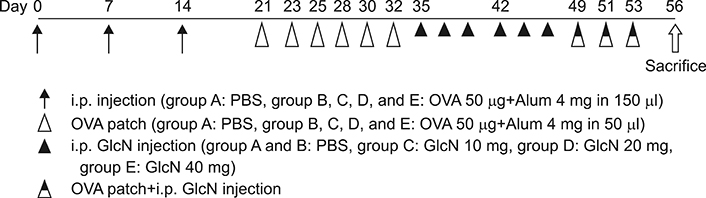
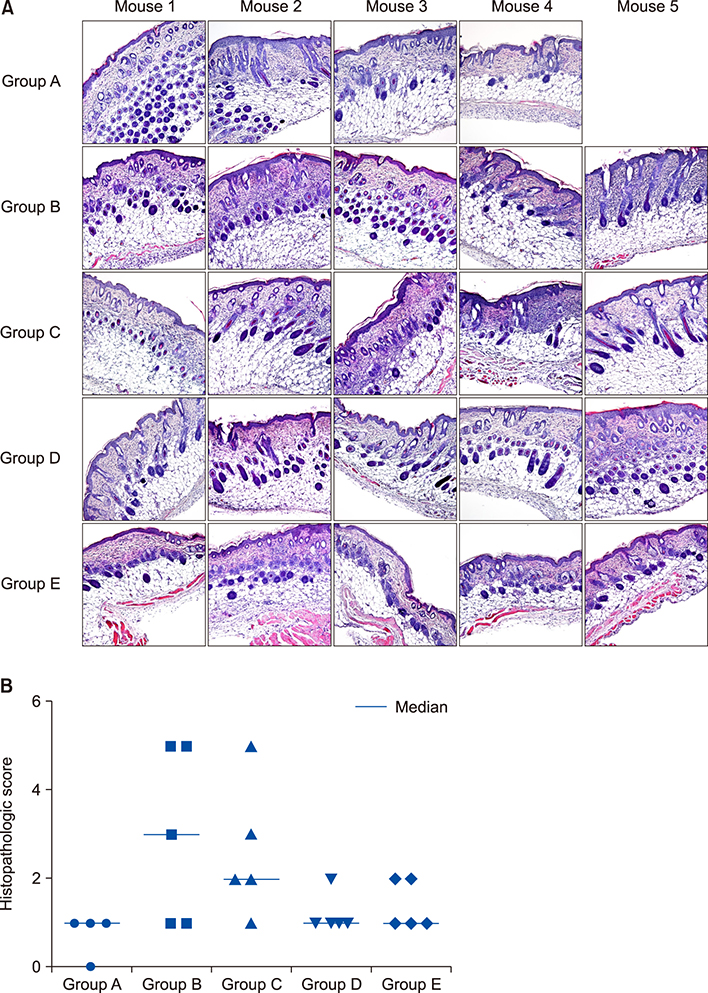
Twenty-five BALB/c mice were divided into five groups (groups A~E). Group A was the phosphate-buffered saline (PBS)-treated group without AD induction. Group B was the PBS control group with AD induction. Groups C to E were the AD induction groups, which were treated with three different doses of GlcN (10 mg, 20 mg, and 40 mg, respectively). Histopathological examination was performed after GlcN administration. Interleukin (IL)-4, IL-13, and IL-17 cytokine levels were measured by enzyme-linked immunosorbent assay using skin biopsy specimens. Serum total immunoglobulin E (IgE) concentrations were measured before and after administration with GlcN or PBS.
RESULTS
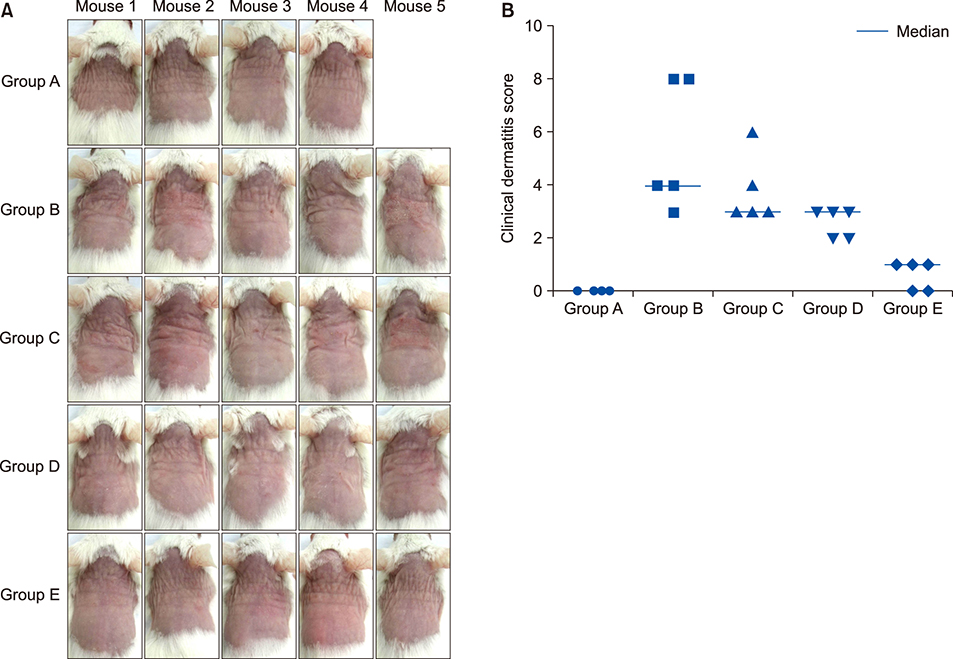
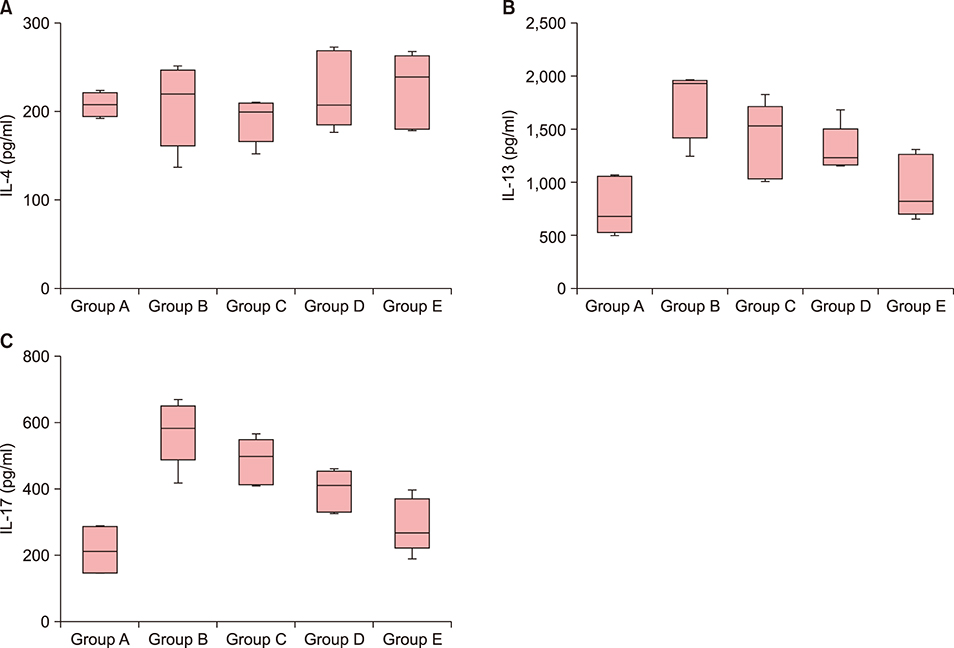
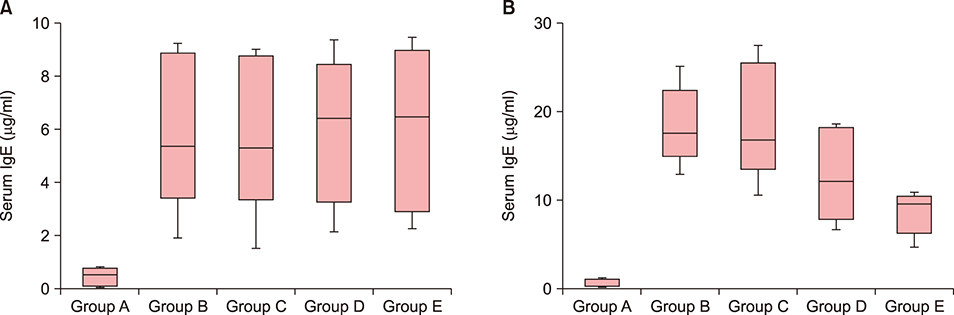
Clinical dermatitis scores decreased with increasing GlcN dose (p<0.001). Concentrations of tissue IL-13 and IL-17 decreased after GlcN administration (each group: p=0.002 and p<0.001, respectively), but the concentrations of tissue IL-4 did not show differences across groups. Serum IgE levels tended to be lower after GlcN administration (p=0.004). Histopathological scores were not significantly different among groups B~E (p=0.394).
CONCLUSION
GlcN improved AD symptoms and decreased tissue IL-13, IL-17, and serum total IgE levels in an animal model.
MeSH Terms
-
Allergy and Immunology
Animals*
Anti-Allergic Agents
Asthma
Biopsy
Dermatitis
Dermatitis, Atopic*
Enzyme-Linked Immunosorbent Assay
Glucosamine*
Immunoglobulin E
Immunoglobulins
Interleukin-13
Interleukin-17
Interleukin-4
Interleukins
Mice
Models, Animal*
Rhinitis
Skin
Therapeutic Uses
Anti-Allergic Agents
Glucosamine
Immunoglobulin E
Immunoglobulins
Interleukin-13
Interleukin-17
Interleukin-4
Interleukins
Therapeutic Uses
Figure
Reference
-
1. Peng W, Novak N. Pathogenesis of atopic dermatitis. Clin Exp Allergy. 2015; 45:566–574.
Article2. Egawa G, Weninger W. Pathogenesis of atopic dermatitis: a short review. Cogent Biol. 2015; 1:1103459.
Article3. Koga C, Kabashima K, Shiraishi N, Kobayashi M, Tokura Y. Possible pathogenic role of Th17 cells for atopic dermatitis. J Invest Dermatol. 2008; 128:2625–2630.
Article4. Vaneckova J, Bukač J. The severity of atopic dermatitis and the relation to the level of total IgE, onset of atopic dermatitis and family history about atopy. Food Agric Immunol. 2016; 27:734–741.
Article5. Sidbury R, Davis DM, Cohen DE, Cordoro KM, Berger TG, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014; 71:327–349.6. Gooderham MJ, Hong HC, Eshtiaghi P, Papp KA. Dupilumab: a review of its use in the treatment of atopic dermatitis. J Am Acad Dermatol. 2018; 78:3 Suppl 1. S28–S36.
Article7. Jung AY, Heo MJ, Kim YH. Glucosamine has an antiallergic effect in mice with allergic asthma and rhinitis. Int Forum Allergy Rhinol. 2017; 7:763–769.
Article8. Nakamura H, Masuko K, Yudoh K, Kato T, Kamada T, Kawahara T. Effects of glucosamine administration on patients with rheumatoid arthritis. Rheumatol Int. 2007; 27:213–218.
Article9. Reginster JY, Deroisy R, Rovati LC, Lee RL, Lejeune E, Bruyere O, et al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet. 2001; 357:251–256.
Article10. Kim CH, Cheong KA, Park CD, Lee AY. Glucosamine improved atopic dermatitis-like skin lesions in NC/Nga mice by inhibition of Th2 cell development. Scand J Immunol. 2011; 73:536–545.
Article11. Jin SE, Jung J, Jun J, Jeon DW, Kim HM, Jeong HJ. Anti-allergic activity of crystallinity controlled N-acetyl glucosamine. Immunopharmacol Immunotoxicol. 2012; 34:991–1000.
Article12. Forchhammer L, Thorn M, Met O, Gad M, Weidner MS, Claesson MH. Immunobiological effects of glucosamine in vitro. Scand J Immunol. 2003; 58:404–411.
Article13. Pauwels R, Bazin H, Platteau B, van der Straeten M. The effect of age on IgE production in rats. Immunology. 1979; 36:145–149.14. Rafi MM, Yadav PN, Rossi AO. Glucosamine inhibits LPS-induced COX-2 and iNOS expression in mouse macrophage cells (RAW 264.7) by inhibition of p38-MAP kinase and transcription factor NF-kappaB. Mol Nutr Food Res. 2007; 51:587–593.
Article15. Hwang JS, Kwon MY, Kim KH, Lee Y, Lyoo IK, Kim JE, et al. Lipopolysaccharide (LPS)-stimulated iNOS induction is increased by glucosamine under normal glucose conditions but Is inhibited by glucosamine under high glucose conditions in macrophage cells. J Biol Chem. 2017; 292:1724–1736.
Article16. Zahedipour F, Dalirfardouei R, Karimi G, Jamialahmadi K. Molecular mechanisms of anticancer effects of Glucosamine. Biomed Pharmacother. 2017; 95:1051–1058.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibitory Effect of Luteolin Liposome Solution by Animal Model for Atopic Dermatitis in NC/Nga Mice
- Measurement of Atopic Dermatitis Disability
- Serum IgE Level in Patients of Atopic Dermatitis and Atopic Dermatitis with Molluscum Contagiosum
- Atopic dermatitis
- A Novel Model for Human Atopic Dermatitis: Application of Repeated DNCB Patch in BALB/c Mice, in Comparison with NC/Nga Mice