Korean J Radiol.
2016 Aug;17(4):509-521. 10.3348/kjr.2016.17.4.509.
Comparison of Multidetector CT and Gadobutrol-Enhanced MR Imaging for Evaluation of Small, Solid Pancreatic Lesions
- Affiliations
-
- 1Department of Radiology, Seoul National University Hospital, Seoul 03080, Korea. jmsh@snu.ac.kr
- 2Institute of Radiation Medicine, Seoul National University Hospital, Seoul 03080, Korea.
- 3Department of Radiology, Konkuk University Medical Center, Seoul 05030, Korea.
- KMID: 2455421
- DOI: http://doi.org/10.3348/kjr.2016.17.4.509
Abstract
OBJECTIVE
To compare multidetector computed tomography (MDCT) and MRI for lesion conspicuity, as well as the detection and characterization of small solid pancreatic lesions (SPLs).
MATERIALS AND METHODS
193 patients with small SPLs (< 3 cm) and 52 patients with normal pancreas who underwent both multiphasic MDCT and gadobutrol-enhanced MRI were included in our study. Two radiologists blinded to the pathologic diagnoses independently reviewed those images, and determined the detection of "SPL per se" and "SPL in consideration of secondary features", the lesion conspicuity, the probability of pancreatic ductal adenocarcinoma (PDAC), and the most likely specific diagnosis.
RESULTS
The sensitivity of MRI for "detection of SPL per se" was significantly higher than that of CT in both reviewers: 92.7% (179/193) and 97.9% (189/193), respectively, for reviewer 1 (p = 0.031) and 90.7% (175/193) and 99.5% (192/193), respectively, for reviewer 2 (p < 0.001). In addition, MRI provided better lesion conspicuity than MDCT for both reviewers (p < 0.001). However, CT and MRI did not show significant difference in sensitivity for "detection of SPL in consideration of secondary features", specificity for SPL detection, and differentiation of PDAC vs. non-PDAC (p > 0.05). The accuracies of CT and MRI for making a specific diagnosis were as follows: 85.7% (210/245) vs. 86.9% (213/245), respectively, for reviewer 1 (p = 0.736), and 91.8% (225/245) vs. 93.5% (229/245), respectively, for reviewer 2 (p = 0.454).
CONCLUSION
MRI showed better lesion conspicuity than MDCT, but did not show significantly different diagnostic performance compared with MDCT for detecting and characterizing small SPLs.
MeSH Terms
-
Adult
Aged
Aged, 80 and over
Area Under Curve
Contrast Media/*chemistry
Databases, Factual
Diagnosis, Differential
Female
Humans
Image Interpretation, Computer-Assisted
*Magnetic Resonance Imaging
Male
Middle Aged
*Multidetector Computed Tomography
Organometallic Compounds/*chemistry
Pancreas/diagnostic imaging
Pancreatic Neoplasms/*diagnosis/diagnostic imaging
ROC Curve
Retrospective Studies
Sensitivity and Specificity
Contrast Media
Organometallic Compounds
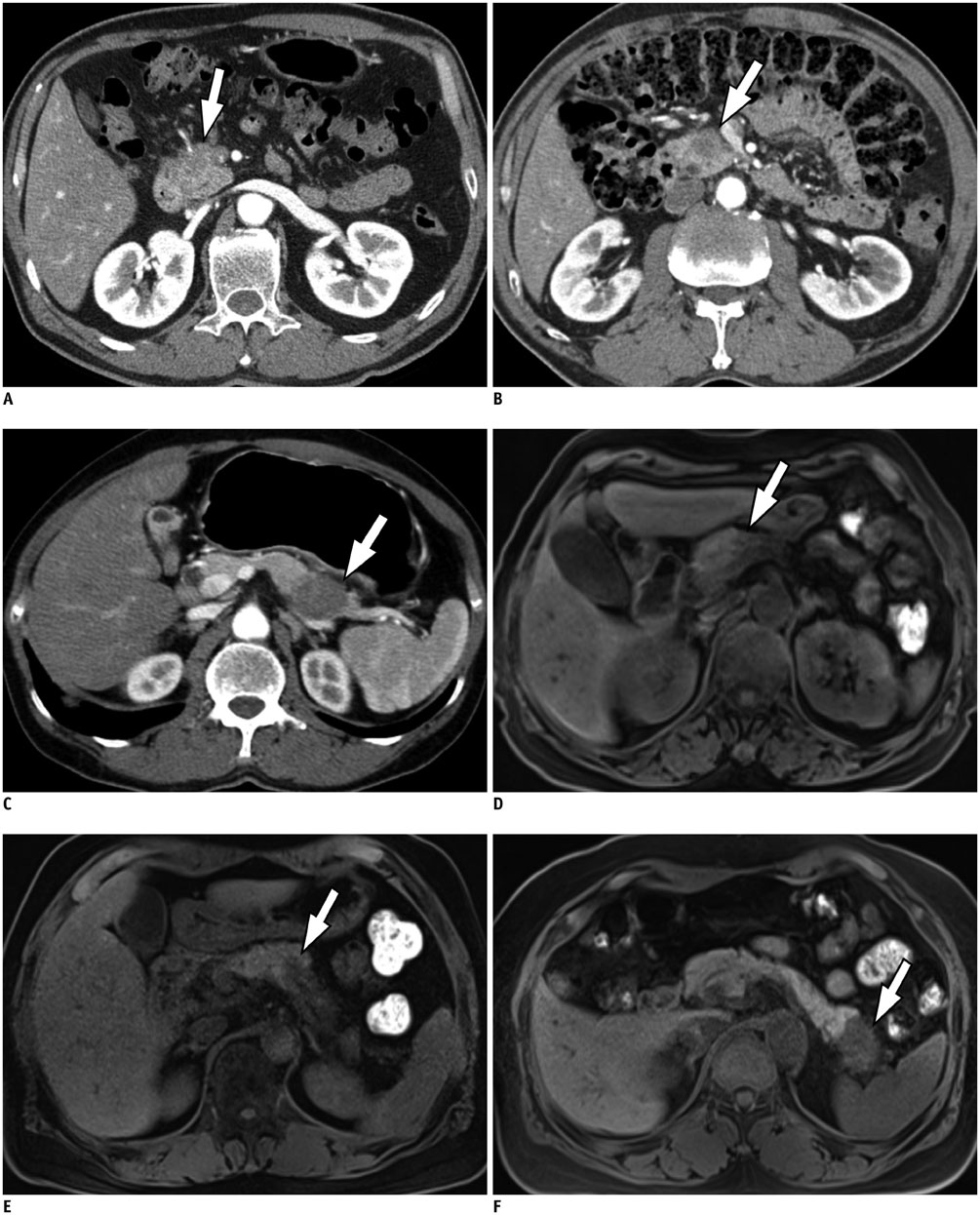
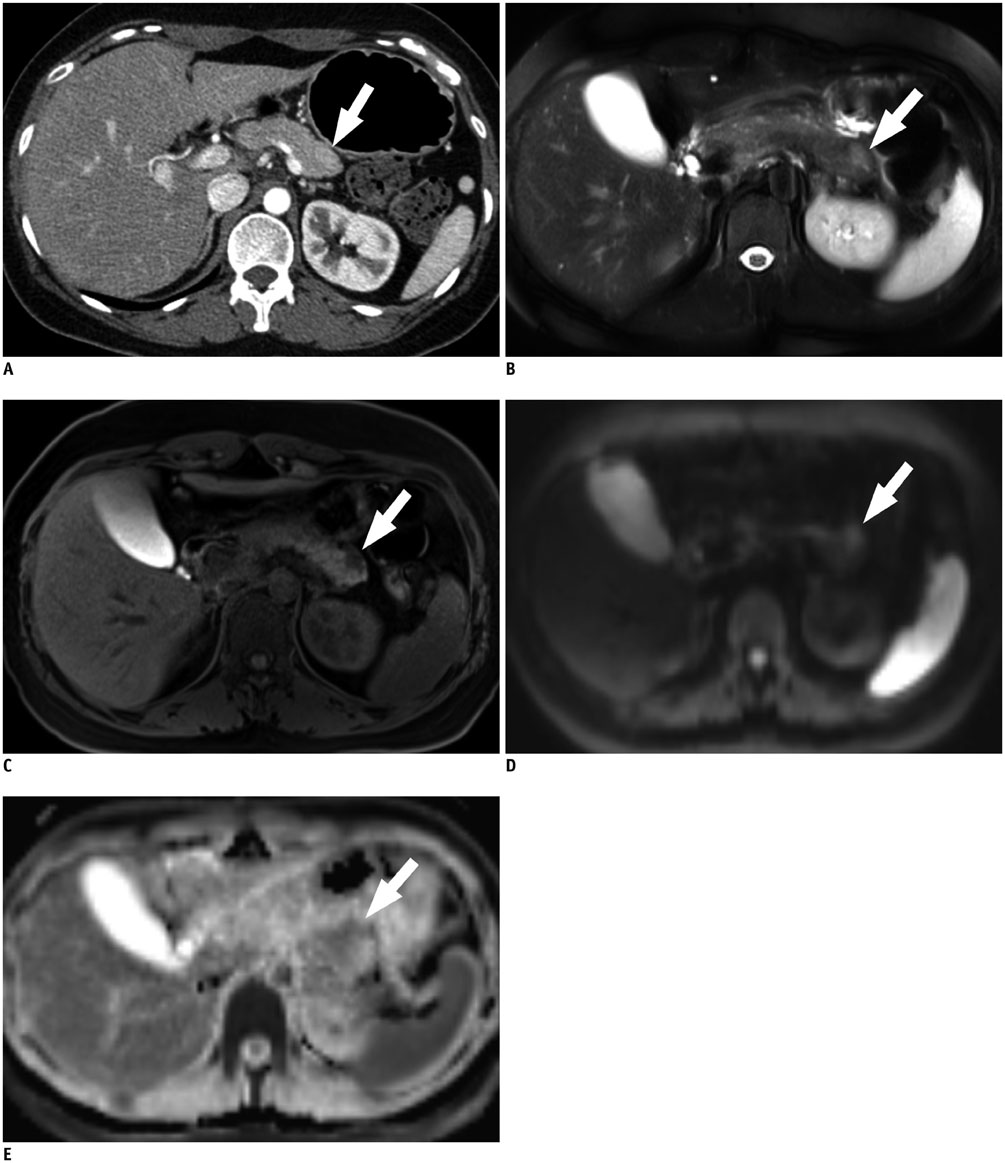
Figure
Reference
-
1. Low G, Panu A, Millo N, Leen E. Multimodality imaging of neoplastic and nonneoplastic solid lesions of the pancreas. Radiographics. 2011; 31:993–1015.2. Saif MW. Pancreatic neoplasm in 2011: an update. JOP. 2011; 12:316–321.3. Tamm EP, Balachandran A, Bhosale PR, Katz MH, Fleming JB, Lee JH, et al. Imaging of pancreatic adenocarcinoma: update on staging/resectability. Radiol Clin North Am. 2012; 50:407–428.4. Al-Hawary MM, Francis IR, Chari ST, Fishman EK, Hough DM, Lu DS, et al. Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the Society of Abdominal Radiology and the American Pancreatic Association. Radiology. 2014; 270:248–260.5. Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg. 1996; 223:273–279.6. Varadhachary GR, Tamm EP, Abbruzzese JL, Xiong HQ, Crane CH, Wang H, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006; 13:1035–1046.7. Kim JH, Lee JM, Park JH, Kim SC, Joo I, Han JK, et al. Solid pancreatic lesions: characterization by using timing bolus dynamic contrast-enhanced MR imaging assessment--a preliminary study. Radiology. 2013; 266:185–196.8. Paspulati RM. Multidetector CT of the pancreas. Radiol Clin North Am. 2005; 43:999–1020. viii9. Tempero MA, Arnoletti JP, Behrman SW, Ben-Josef E, Benson AB 3rd, Casper ES, et al. Pancreatic Adenocarcinoma, version 2.2012: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2012; 10:703–713.10. Yoon SH, Lee JM, Cho JY, Lee KB, Kim JE, Moon SK, et al. Small (≤ 20 mm) pancreatic adenocarcinomas: analysis of enhancement patterns and secondary signs with multiphasic multidetector CT. Radiology. 2011; 259:442–452.11. Blouhos K, Boulas KA, Tselios DG, Katsaouni SP, Mauroeidi B, Hatzigeorgiadis A. Surgically proved visually isoattenuating pancreatic adenocarcinoma undetected in both dynamic CT and MRI. Was blind pancreaticoduodenectomy justified? Int J Surg Case Rep. 2013; 4:466–469.12. Kim JH, Park SH, Yu ES, Kim MH, Kim J, Byun JH, et al. Visually isoattenuating pancreatic adenocarcinoma at dynamic-enhanced CT: frequency, clinical and pathologic characteristics, and diagnosis at imaging examinations. Radiology. 2010; 257:87–96.13. Kang KM, Lee JM, Yoon JH, Kiefer B, Han JK, Choi BI. Intravoxel incoherent motion diffusion-weighted MR imaging for characterization of focal pancreatic lesions. Radiology. 2014; 270:444–453.14. Yu MH, Lee JY, Kim MA, Kim SH, Lee JM, Han JK, et al. MR imaging features of small solid pseudopapillary tumors: retrospective differentiation from other small solid pancreatic tumors. AJR Am J Roentgenol. 2010; 195:1324–1332.15. Jang KM, Kim SH, Kim YK, Park MJ, Lee MH, Hwang J, et al. Imaging features of small (≤ 3 cm) pancreatic solid tumors on gadoxetic-acid-enhanced MR imaging and diffusion-weighted imaging: an initial experience. Magn Reson Imaging. 2012; 30:916–925.16. Hur BY, Lee JM, Lee JE, Park JY, Kim SJ, Joo I, et al. Magnetic resonance imaging findings of the mass-forming type of autoimmune pancreatitis: comparison with pancreatic adenocarcinoma. J Magn Reson Imaging. 2012; 36:188–197.17. Rao SX, Zeng MS, Cheng WZ, Yao XZ, Jin DY, Ji Y. Small solid tumors (< or = 2 cm) of the pancreas: relative accuracy and differentiation of CT and MR imaging. Hepatogastroenterology. 2011; 58:996–1001.18. Otsuki M, Chung JB, Okazaki K, Kim MH, Kamisawa T, Kawa S, et al. Asian diagnostic criteria for autoimmune pancreatitis: consensus of the Japan-Korea Symposium on Autoimmune Pancreatitis. J Gastroenterol. 2008; 43:403–408.19. Baek JH, Lee JM, Kim SH, Kim SJ, Kim SH, Lee JY, et al. Small (<or=3 cm) solid pseudopapillary tumors of the pancreas at multiphasic multidetector CT. Radiology. 2010; 257:97–106.20. Lu DS, Vedantham S, Krasny RM, Kadell B, Berger WL, Reber HA. Two-phase helical CT for pancreatic tumors: pancreatic versus hepatic phase enhancement of tumor, pancreas, and vascular structures. Radiology. 1996; 199:697–701.21. Haradome H, Grazioli L, Tsunoo M, Tinti R, Frittoli B, Gambarini S, et al. Can MR fluoroscopic triggering technique and slow rate injection provide appropriate arterial phase images with reducing artifacts on gadoxetic acid-DTPA (Gd-EOB-DTPA)-enhanced hepatic MR imaging? J Magn Reson Imaging. 2010; 32:334–340.22. Hussain HK, Londy FJ, Francis IR, Nghiem HV, Weadock WJ, Gebremariam A, et al. Hepatic arterial phase MR imaging with automated bolus-detection three-dimensional fast gradient-recalled-echo sequence: comparison with test-bolus method. Radiology. 2003; 226:558–566.23. Park SH, Goo JM, Jo CH. Receiver operating characteristic (ROC) curve: practical review for radiologists. Korean J Radiol. 2004; 5:11–18.24. Kim SY, Park SH, Hong N, Kim JH, Hong SM. Primary solid pancreatic tumors: recent imaging findings updates with pathology correlation. Abdom Imaging. 2013; 38:1091–1105.25. Sahani DV, Shah ZK, Catalano OA, Boland GW, Brugge WR. Radiology of pancreatic adenocarcinoma: current status of imaging. J Gastroenterol Hepatol. 2008; 23:23–33.26. Rha SE, Jung SE, Lee KH, Ku YM, Byun JY, Lee JM. CT and MR imaging findings of endocrine tumor of the pancreas according to WHO classification. Eur J Radiol. 2007; 62:371–377.27. Ganeshan DM, Paulson E, Tamm EP, Taggart MW, Balachandran A, Bhosale P. Solid pseudo-papillary tumors of the pancreas: current update. Abdom Imaging. 2013; 38:1373–1382.28. Ferrozzi F, Bova D, Campodonico F, Chiara FD, Passari A, Bassi P. Pancreatic metastases: CT assessment. Eur Radiol. 1997; 7:241–245.29. Klein KA, Stephens DH, Welch TJ. CT characteristics of metastatic disease of the pancreas. Radiographics. 1998; 18:369–378.30. Ng CS, Loyer EM, Iyer RB, David CL, DuBrow RA, Charnsangavej C. Metastases to the pancreas from renal cell carcinoma: findings on three-phase contrast-enhanced helical CT. AJR Am J Roentgenol. 1999; 172:1555–1559.31. Palmowski M, Hacke N, Satzl S, Klauss M, Wente MN, Neukamm M, et al. Metastasis to the pancreas: characterization by morphology and contrast enhancement features on CT and MRI. Pancreatology. 2008; 8:199–203.32. Lee JH, Byun JH, Kim JH, Lee SS, Kim HJ, Lee MG. Solid pancreatic tumors with unilocular cyst-like appearance on CT: differentiation from unilocular cystic tumors using CT. Korean J Radiol. 2014; 15:704–711.33. Rosenkrantz AB, Lee L, Matza BW, Kim S. Infiltrative hepatocellular carcinoma: comparison of MRI sequences for lesion conspicuity. Clin Radiol. 2012; 67:e105–e111.34. Miller FH, Rini NJ, Keppke AL. MRI of adenocarcinoma of the pancreas. AJR Am J Roentgenol. 2006; 187:W365–W374.35. Takakura K, Sumiyama K, Munakata K, Ashida H, Arihiro S, Kakutani H, et al. Clinical usefulness of diffusion-weighted MR imaging for detection of pancreatic cancer: comparison with enhanced multidetector-row CT. Abdom Imaging. 2011; 36:457–462.36. Park HS, Lee JM, Choi HK, Hong SH, Han JK, Choi BI. Preoperative evaluation of pancreatic cancer: comparison of gadolinium-enhanced dynamic MRI with MR cholangiopancreatography versus MDCT. J Magn Reson Imaging. 2009; 30:586–595.37. Vachiranubhap B, Kim YH, Balci NC, Semelka RC. Magnetic resonance imaging of adenocarcinoma of the pancreas. Top Magn Reson Imaging. 2009; 20:3–9.38. Ichikawa T, Erturk SM, Motosugi U, Sou H, Iino H, Araki T, et al. High-b value diffusion-weighted MRI for detecting pancreatic adenocarcinoma: preliminary results. AJR Am J Roentgenol. 2007; 188:409–414.39. Prokesch RW, Chow LC, Beaulieu CF, Bammer R, Jeffrey RB Jr. Isoattenuating pancreatic adenocarcinoma at multi-detector row CT: secondary signs. Radiology. 2002; 224:764–768.40. Ishigami K, Yoshimitsu K, Irie H, Tajima T, Asayama Y, Nishie A, et al. Diagnostic value of the delayed phase image for iso-attenuating pancreatic carcinomas in the pancreatic parenchymal phase on multidetector computed tomography. Eur J Radiol. 2009; 69:139–146.41. Zamboni GA, Bernardin L, Pozzi Mucelli R. Dynamic MDCT of the pancreas: is time-density curve morphology useful for the differential diagnosis of solid lesions? A preliminary report. Eur J Radiol. 2012; 81:e381–e385.42. Kitano M, Kudo M, Yamao K, Takagi T, Sakamoto H, Komaki T, et al. Characterization of small solid tumors in the pancreas: the value of contrast-enhanced harmonic endoscopic ultrasonography. Am J Gastroenterol. 2012; 107:303–310.43. Park HS, Lee JM, Choi JY, Lee MW, Kim HJ, Han JK, et al. Preoperative evaluation of bile duct cancer: MRI combined with MR cholangiopancreatography versus MDCT with direct cholangiography. AJR Am J Roentgenol. 2008; 190:396–405.44. Sainani NI, Saokar A, Deshpande V, Fernández-del Castillo C, Hahn P, Sahani DV. Comparative performance of MDCT and MRI with MR cholangiopancreatography in characterizing small pancreatic cysts. AJR Am J Roentgenol. 2009; 193:722–731.45. Schmid-Tannwald C, Oto A, Reiser MF, Zech CJ. Diffusion-weighted MRI of the abdomen: current value in clinical routine. J Magn Reson Imaging. 2013; 37:35–47.46. Matsuki M, Inada Y, Nakai G, Tatsugami F, Tanikake M, Narabayashi I, et al. Diffusion-weighed MR imaging of pancreatic carcinoma. Abdom Imaging. 2007; 32:481–483.47. Kim H, Lee JM, Yoon JH, Jang JY, Kim SW, Ryu JK, et al. Reduced field-of-view diffusion-weighted magnetic resonance imaging of the pancreas: comparison with conventional single-shot echo-planar imaging. Korean J Radiol. 2015; 16:1216–1225.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of contrast-enhanced harmonic endoscopic ultrasonography (EUS) and EUS elastography in pancreatic lesions
- Differential Diagnosis of Pancreatic Cancer and its Mimicking Lesions
- Pancreatic Tumors: Emphasis on CT Findings and Pathologic Classification
- Pancreas Neuroendocrine Tumor and Its Mimics: Review of Cross-Sectional Imaging Findings for Differential Diagnosis
- Contrast-enhanced endoscopic ultrasound for pancreatobiliary disease