Cancer Res Treat.
2019 Jul;51(3):1144-1155. 10.4143/crt.2018.508.
Development of Web-Based Nomograms to Predict Treatment Response and Prognosis of Epithelial Ovarian Cancer
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Seoul National University College of Medicine, Seoul, Korea. yssong@snu.ac.kr
- 2Department of Statistics, The Research Institute of Natural Sciences, Sookmyung Women’s University, Seoul, Korea.
- 3Department of Statistics, Seoul National University, Seoul, Korea. tspark@stats.snu.ac.kr
- 4Center for Precision Medicine, Seoul National University Hospital, Seoul, Korea.
- 5Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 6Interdisciplinary Program in Cancer Biology, Seoul National University College of Medicine, Seoul, Korea.
- 7Department of Obstetrics and Gynecology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2454306
- DOI: http://doi.org/10.4143/crt.2018.508
Abstract
- PURPOSE
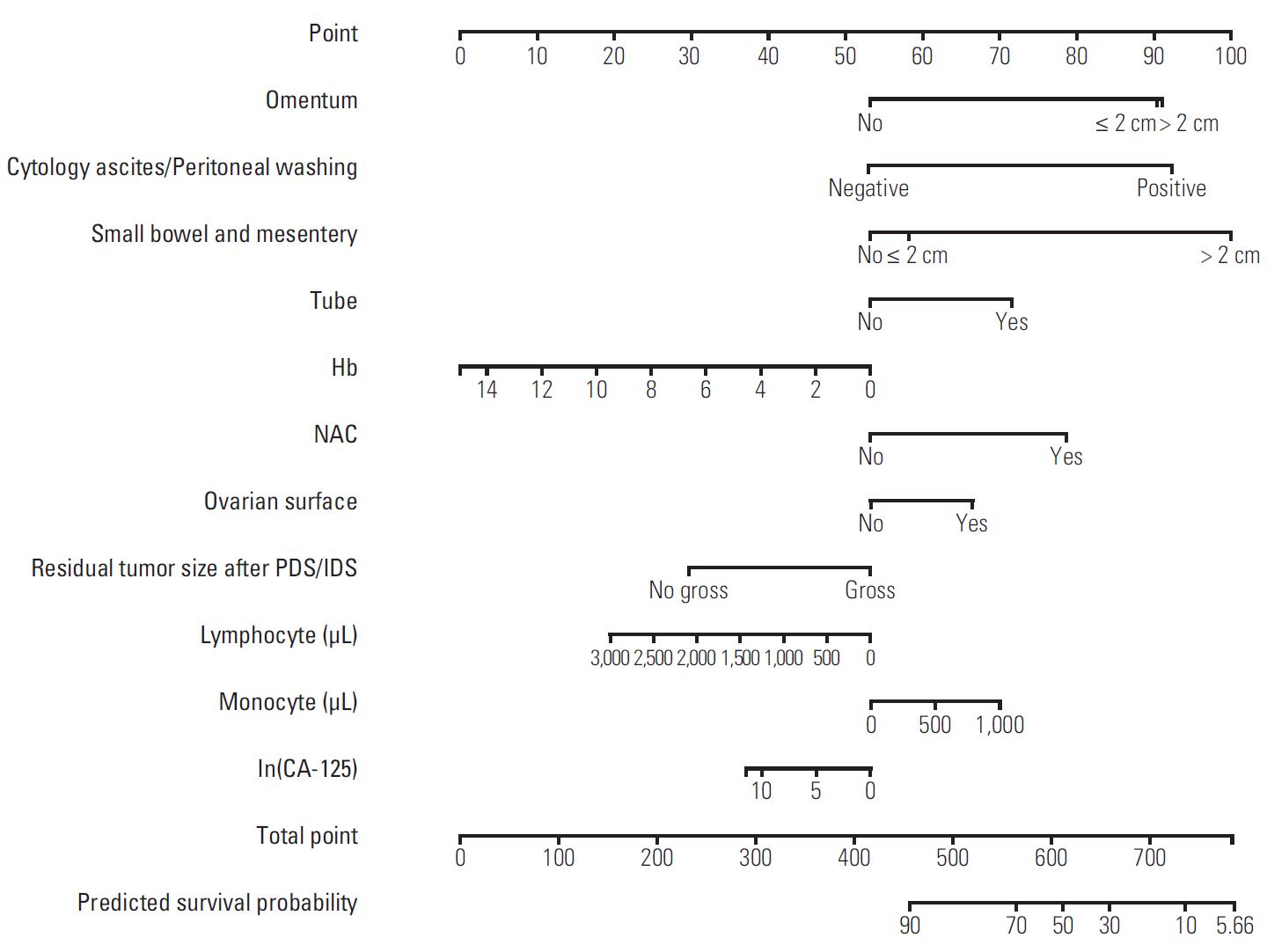
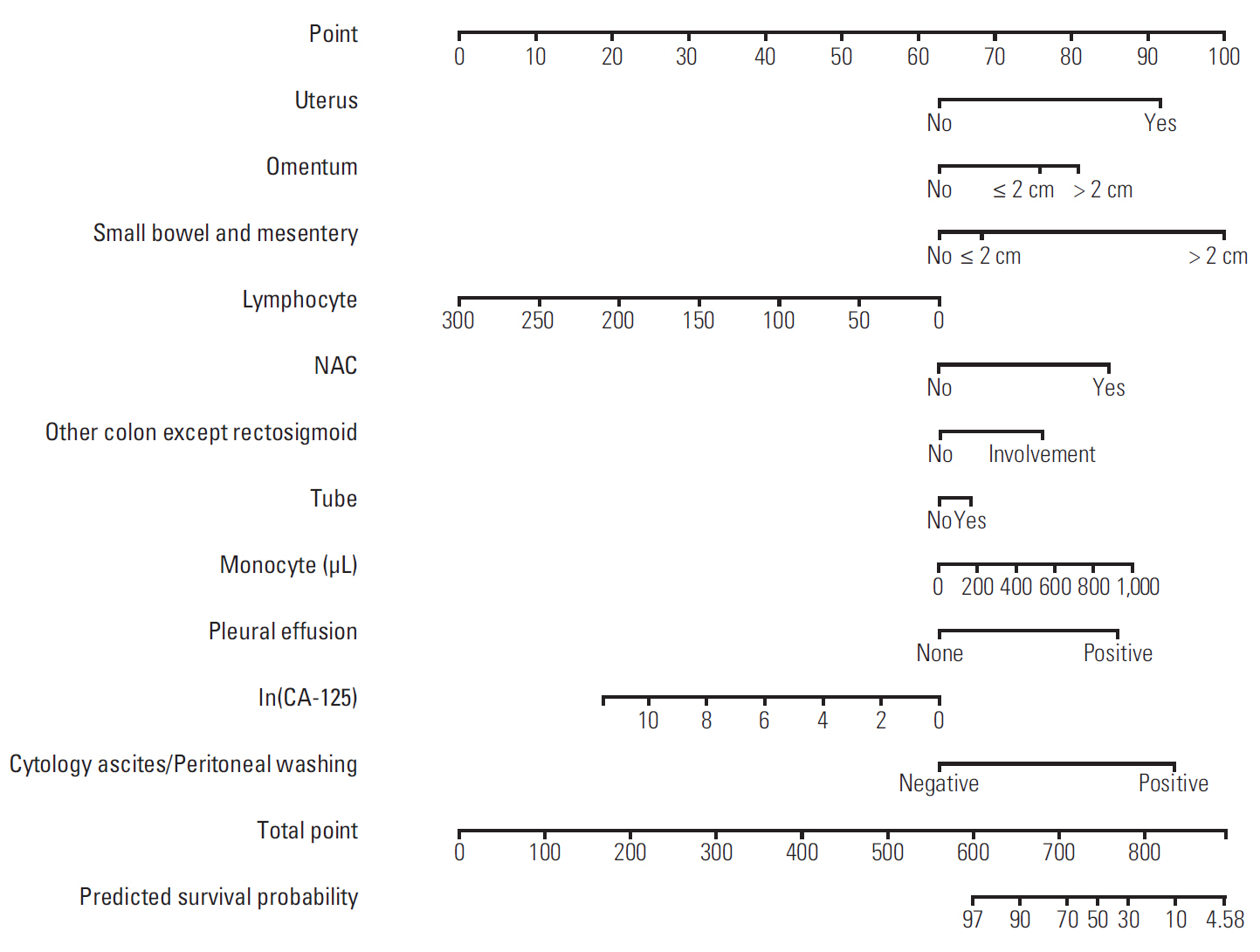
Discovery of models predicting the exact prognosis of epithelial ovarian cancer (EOC) is necessary as the first step of implementation of individualized treatment. This study aimed to develop nomograms predicting treatment response and prognosis in EOC.
MATERIALS AND METHODS
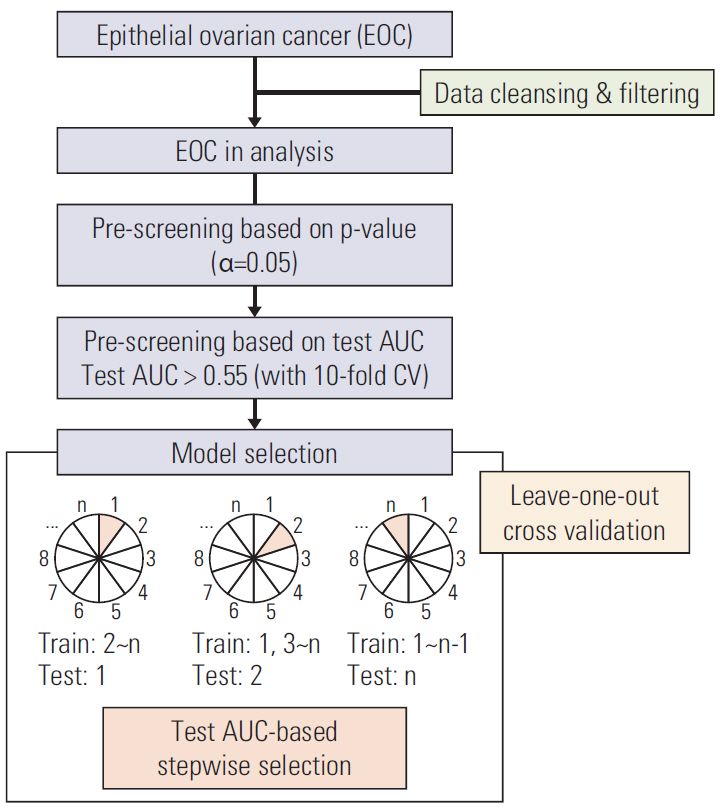
We comprehensively reviewed medical records of 866 patients diagnosed with and treated for EOC at two tertiary institutional hospitals between 2007 and 2016. Patients' clinico-pathologic characteristics, details of primary treatment, intra-operative surgical findings, and survival outcomes were collected. To construct predictive nomograms for platinum sensitivity, 3-year progression-free survival (PFS), and 5-year overall survival (OS), we performed stepwise variable selection by measuring the area under the receiver operating characteristic curve (AUC) with leave-one-out cross-validation. For model validation, 10-fold cross-validation was applied.
RESULTS
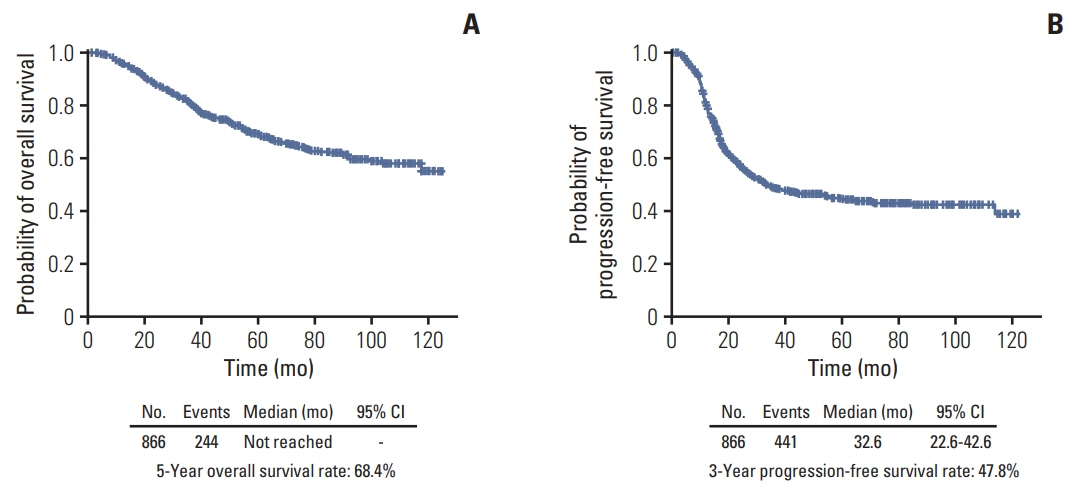
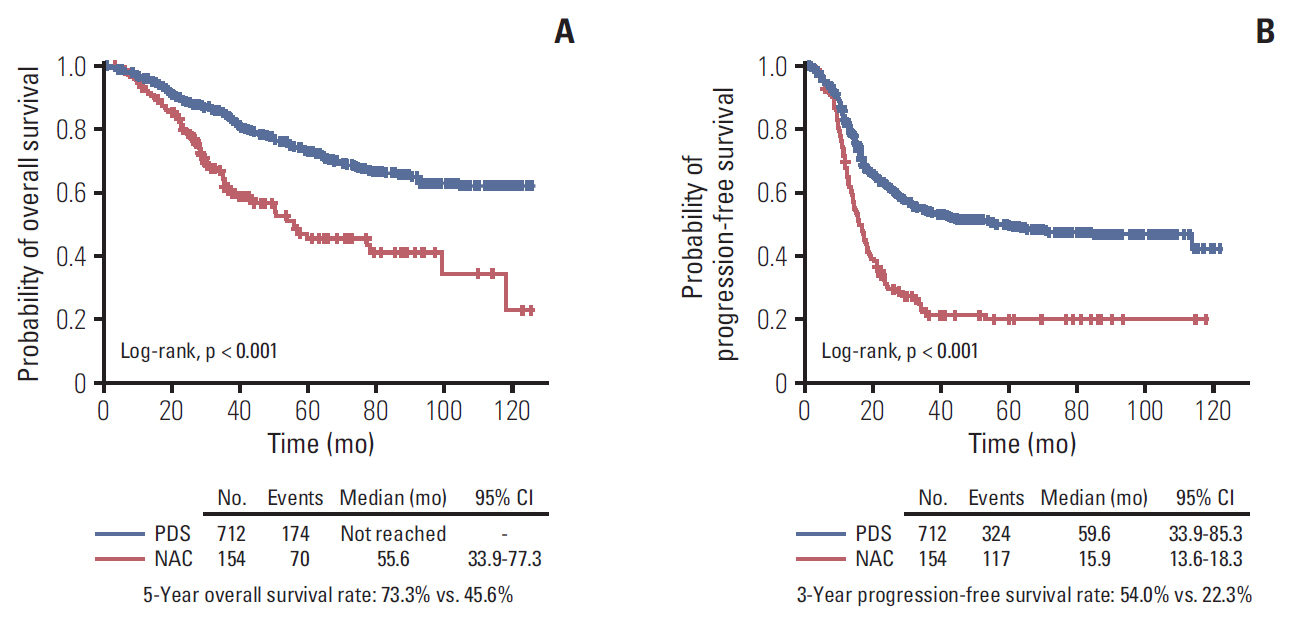
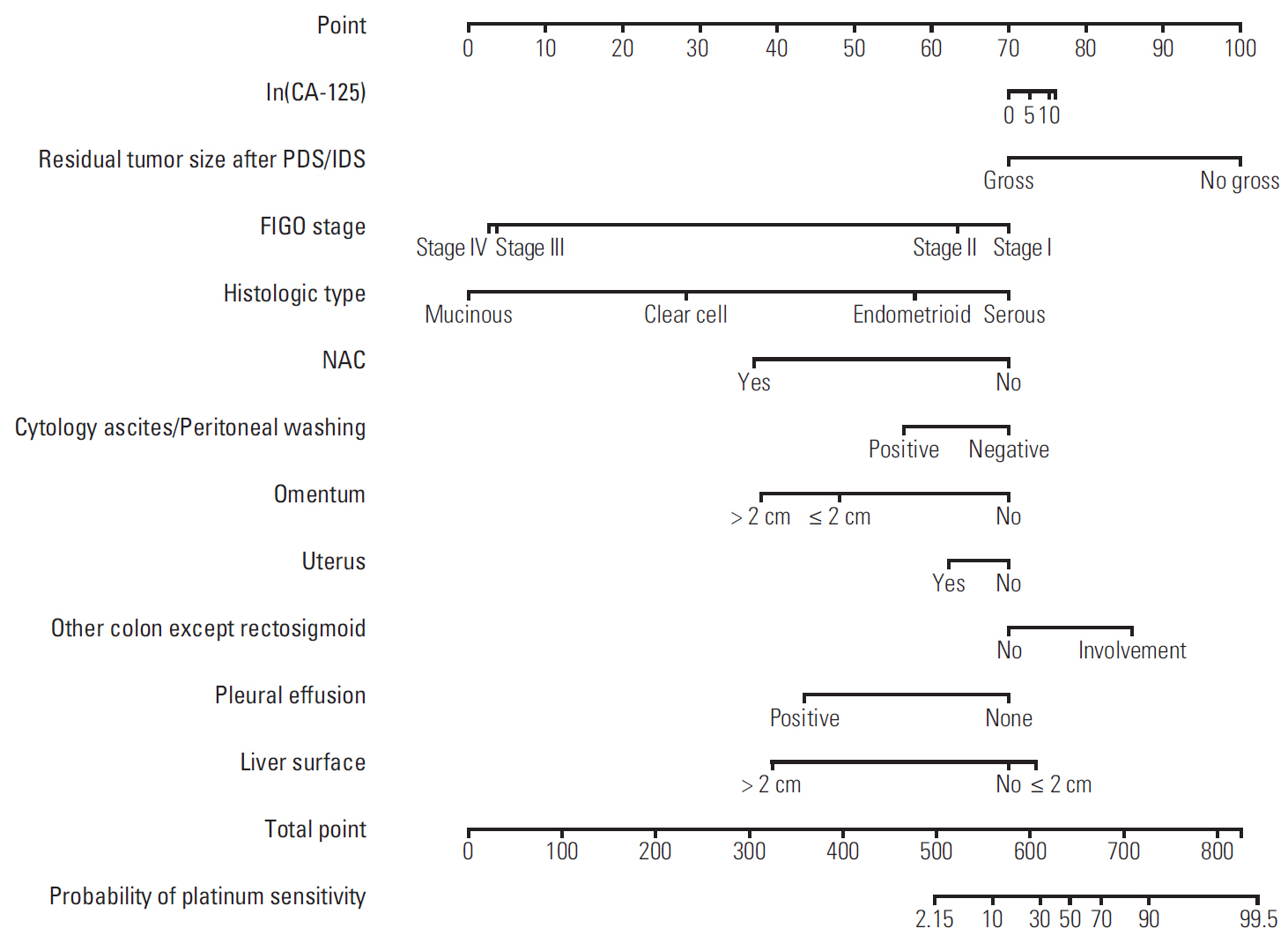
The median length of observation was 42.4 months (interquartile range, 25.7 to 69.9 months), during which 441 patients (50.9%) experienced disease recurrence. The median value of PFS was 32.6 months and 3-year PFS rate was 47.8% while 5-year OS rate was 68.4%. The AUCs of the newly developed nomograms predicting platinum sensitivity, 3-year PFS, and 5-year OS were 0.758, 0.841, and 0.805, respectively. We also developed predictive nomograms confined to the patients who underwent primary debulking surgery. The AUCs for platinum sensitivity, 3-year PFS, and 5-year OS were 0.713, 0.839, and 0.803, respectively.
CONCLUSION
We successfully developed nomograms predicting treatment response and prognosis of patients with EOC. These nomograms are expected to be useful in clinical practice and designing clinical trials.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017; 67:7–30.
Article2. Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Kosary CL, et al. SEER cancer statistics review, 1975-2014 [Internet]. Bethesda, MD: National Cancer Institute;2017. [cited 2018 May 10]. Available from: https://seer.cancer.gov/csr/1975_2014/.3. Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, et al. The global burden of cancer 2013. JAMA Oncol. 2015; 1:505–27.4. Lim MC, Moon EK, Shin A, Jung KW, Won YJ, Seo SS, et al. Incidence of cervical, endometrial, and ovarian cancer in Korea, 1999-2010. J Gynecol Oncol. 2013; 24:298–302.
Article5. Smith LH, Morris CR, Yasmeen S, Parikh-Patel A, Cress RD, Romano PS. Ovarian cancer: can we make the clinical diagnosis earlier? Cancer. 2005; 104:1398–407.
Article6. Cho KR, Shih Ie IM. Ovarian cancer. Annu Rev Pathol. 2009; 4:287–313.
Article7. Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002; 20:1248–59.
Article8. Chi DS, Franklin CC, Levine DA, Akselrod F, Sabbatini P, Jarnagin WR, et al. Improved optimal cytoreduction rates for stages IIIC and IV epithelial ovarian, fallopian tube, and primary peritoneal cancer: a change in surgical approach. Gynecol Oncol. 2004; 94:650–4.
Article9. Wimberger P, Wehling M, Lehmann N, Kimmig R, Schmalfeldt B, Burges A, et al. Influence of residual tumor on outcome in ovarian cancer patients with FIGO stage IV disease: an exploratory analysis of the AGO-OVAR (Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group). Ann Surg Oncol. 2010; 17:1642–8.10. Cannistra SA. Cancer of the ovary. N Engl J Med. 2004; 351:2519–29.
Article11. Teramukai S, Ochiai K, Tada H, Fukushima M; Japan Multinational Trial Organization OC01-01. PIEPOC: a new prognostic index for advanced epithelial ovarian cancer--Japan Multinational Trial Organization OC01-01. J Clin Oncol. 2007; 25:3302–6.
Article12. Chi DS, Palayekar MJ, Sonoda Y, Abu-Rustum NR, Awtrey CS, Huh J, et al. Nomogram for survival after primary surgery for bulky stage IIIC ovarian carcinoma. Gynecol Oncol. 2008; 108:191–4.
Article13. Gerestein CG, Eijkemans MJ, de Jong D, van der Burg ME, Dykgraaf RH, Kooi GS, et al. The prediction of progression-free and overall survival in women with an advanced stage of epithelial ovarian carcinoma. BJOG. 2009; 116:372–80.
Article14. Barlin JN, Yu C, Hill EK, Zivanovic O, Kolev V, Levine DA, et al. Nomogram for predicting 5-year disease-specific mortality after primary surgery for epithelial ovarian cancer. Gynecol Oncol. 2012; 125:25–30.
Article15. Lee CK, Simes RJ, Brown C, Lord S, Wagner U, Plante M, et al. Prognostic nomogram to predict progression-free survival in patients with platinum-sensitive recurrent ovarian cancer. Br J Cancer. 2011; 105:1144–50.
Article16. Lee CK, Simes RJ, Brown C, Gebski V, Pfisterer J, Swart AM, et al. A prognostic nomogram to predict overall survival in patients with platinum-sensitive recurrent ovarian cancer. Ann Oncol. 2013; 24:937–43.
Article17. Previs RA, Bevis KS, Huh W, Tillmanns T, Perry L, Moore K, et al. A prognostic nomogram to predict overall survival in women with recurrent ovarian cancer treated with bevacizumab and chemotherapy. Gynecol Oncol. 2014; 132:531–6.
Article18. Paik ES, Sohn I, Baek SY, Shim M, Choi HJ, Kim TJ, et al. Nomograms predicting platinum sensitivity, progression-free survival, and overall survival using pretreatment complete blood cell counts in epithelial ovarian cancer. Cancer Res Treat. 2017; 49:635–42.
Article19. Foote J, Lopez-Acevedo M, Samsa G, Lee PS, Kamal AH, Alvarez Secord A, et al. Predicting 6- and 12-month risk of mortality in patients with platinum-resistant advanced-stage ovarian cancer: prognostic model to guide palliative care referrals. Int J Gynecol Cancer. 2018; 28:302–7.20. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–47.
Article21. Rustin GJ, Vergote I, Eisenhauer E, Pujade-Lauraine E, Quinn M, Thigpen T, et al. Definitions for response and progression in ovarian cancer clinical trials incorporating RECIST 1.1 and CA 125 agreed by the Gynecological Cancer Intergroup (GCIG). Int J Gynecol Cancer. 2011; 21:419–23.
Article22. Heagerty PJ, Zheng Y. Survival model predictive accuracy and ROC curves. Biometrics. 2005; 61:92–105.
Article23. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008; 454:436–44.
Article24. Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol. 2014; 32:1302–8.
Article25. Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012; 30:2039–45.
Article26. Coleman RL, Brady MF, Herzog TJ, Sabbatini P, Armstrong DK, Walker JL, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017; 18:779–91.
Article27. Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017; 18:1274–84.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Development and Validation of Web-Based Nomograms to Precisely Predict Survival Outcomes of Non-metastatic Nasopharyngeal Carcinoma in an Endemic Area
- A Case of Recurrent Early-stage Epithelial Ovarian Cancer Presenting as Bone Metastasis
- Quantitative Pathologic Variables as Prognostic Factors in Epithelial Ovarian Cancer
- Nomograms Predicting Platinum Sensitivity, Progression-Free Survival, and Overall Survival Using Pretreatment Complete Blood Cell Counts in Epithelial Ovarian Cancer
- How to predict treatment failure in frail patients with advanced epithelial ovarian cancer: strategies to personalize surgical effort