Cancer Res Treat.
2019 Jul;51(3):1073-1085. 10.4143/crt.2018.357.
A Nomogram for Predicting the Oncotype DX Recurrence Score in Women with T1-3N0-1miM0 Hormone Receptor‒Positive, Human Epidermal Growth Factor 2 (HER2)‒Negative Breast Cancer
- Affiliations
-
- 1Division of Breast Surgery, Department of Surgery, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea. jjjongwr@hanmail.net
- 2Samsung Electronics, Giheung/Hwaseong/Pyeongtaek Complex, Smart IT team, Hwaseong, Korea.
- 3Department of Oncology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea. khjung@amc.seoul.kr
- KMID: 2454299
- DOI: http://doi.org/10.4143/crt.2018.357
Abstract
- PURPOSE
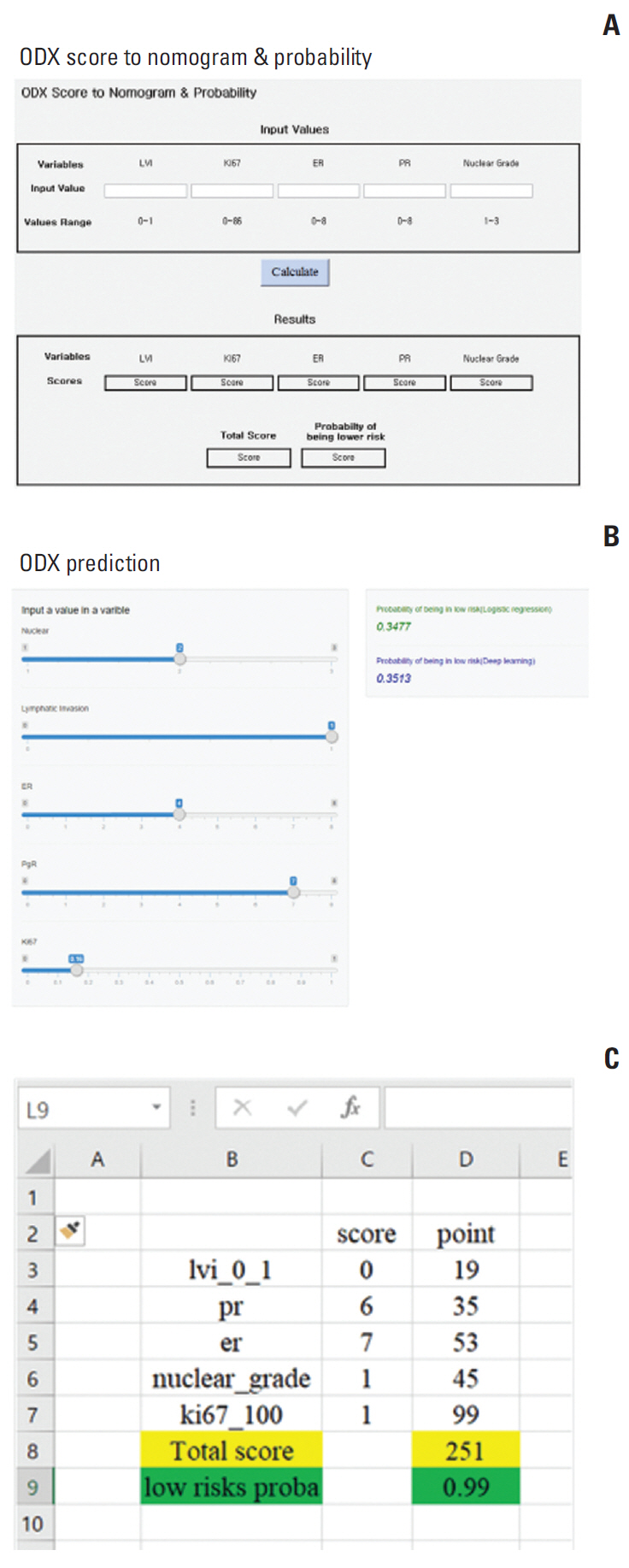
This preliminary study was conducted to evaluate the association between Oncotype DX (ODX) recurrence score and traditional prognostic factors. We also developed a nomogram to predict subgroups with low ODX recurrence scores (less than 25) and to avoid additional chemotherapy treatments for those patients.
MATERIALS AND METHODS
Clinicopathological and immunohistochemical variables were retrospectively retrieved and analyzed from a series of 485 T1-3N0-1miM0 hormone receptor-positive, human epidermal growth factor 2"’negative breast cancer patients with available ODX test results at Asan Medical Center from 2010 to 2016. One hundred twenty-seven patients (26%) had positive axillary lymph node micrometastases, and 408 (84%) had ODX recurrence scores of ≤25. Logistic regression was performed to build a nomogram for predicting a low-risk subgroup of the ODX assay.
RESULTS
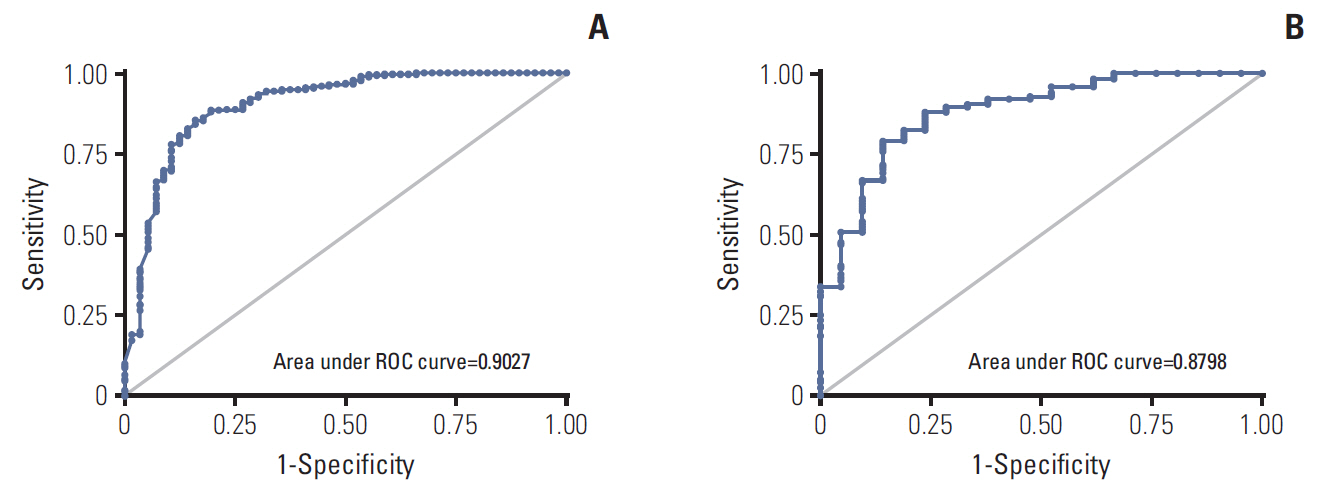
Multivariate analysis revealed that estrogen receptor (ER) score, progesterone receptor (PR) score, histologic grade, lymphovascular invasion (LVI), and Ki-67 had a statistically significant association with the low-risk subgroup. With these variables, we developed a nomogram to predict the low-risk subgroup with ODX recurrence scores of ≤25. The area under the receiver operating characteristic curve was 0.90 (95% confidence interval [CI], 0.85 to 0.96). When applied to the validation group the nomogram was accurate with an area under the curve = 0.88 (95% CI, 0.83 to 0.95).
CONCLUSION
The low ODX recurrence score subgroup can be predicted by a nomogram incorporating five traditional prognostic factors: ER, PR, histologic grade, LVI, and Ki-67. Our nomogram, which predicts a low-risk ODX recurrence score, will be a useful tool to help select patients who may or may not need additional ODX testing.
Keyword
MeSH Terms
-
Breast Neoplasms*
Breast*
Chungcheongnam-do
Drug Therapy
Epidermal Growth Factor*
Estrogens
Female
Humans
Humans*
Logistic Models
Lymph Nodes
Multivariate Analysis
Neoplasm Micrometastasis
Nomograms*
Prognosis
Receptors, Progesterone
Recurrence*
Retrospective Studies
ROC Curve
Epidermal Growth Factor
Estrogens
Receptors, Progesterone
Figure
Cited by 1 articles
-
A simplified risk scoring system for predicting high-risk groups in gene expression tests for patients with estrogen receptor-positive, human epidermal growth factor receptor 2-negative, and node-positive breast cancer
Kwang Hyun Yoon, Suk Jun Lee, Yujin Kim, Jee Hyun Ahn, Jee Ye Kim, Hyung Seok Park, Seung Il Kim, Seho Park
Ann Surg Treat Res. 2023;105(6):360-368. doi: 10.4174/astr.2023.105.6.360.
Reference
-
References
1. Rosenberg J, Chia YL, Plevritis S. The effect of age, race, tumor size, tumor grade, and disease stage on invasive ductal breast cancer survival in the U.S. SEER database. Breast Cancer Res Treat. 2005; 89:47–54.
Article2. Koscielny S, Tubiana M, Le MG, Valleron AJ, Mouriesse H, Contesso G, et al. Breast cancer: relationship between the size of the primary tumour and the probability of metastatic dissemination. Br J Cancer. 1984; 49:709–15.
Article3. Fisher B, Bauer M, Wickerham DL, Redmond CK, Fisher ER, Cruz AB, et al. Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer. An NSABP update. Cancer. 1983; 52:1551–7.
Article4. Baak JP, Gudlaugsson E, Skaland I, Guo LH, Klos J, Lende TH, et al. Proliferation is the strongest prognosticator in node-negative breast cancer: significance, error sources, alternatives and comparison with molecular prognostic markers. Breast Cancer Res Treat. 2009; 115:241–54.
Article5. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 2002; 41:154–61.
Article6. Colditz GA, Rosner BA, Chen WY, Holmes MD, Hankinson SE. Risk factors for breast cancer according to estrogen and progesterone receptor status. J Natl Cancer Inst. 2004; 96:218–28.
Article7. Joh JE, Esposito NN, Kiluk JV, Laronga C, Lee MC, Loftus L, et al. The effect of Oncotype DX recurrence score on treatment recommendations for patients with estrogen receptor-positive early stage breast cancer and correlation with estimation of recurrence risk by breast cancer specialists. Oncologist. 2011; 16:1520–6.
Article8. Rouzier R, Pronzato P, Chereau E, Carlson J, Hunt B, Valentine WJ. Multigene assays and molecular markers in breast cancer: systematic review of health economic analyses. Breast Cancer Res Treat. 2013; 139:621–37.
Article9. Klein ME, Dabbs DJ, Shuai Y, Brufsky AM, Jankowitz R, Puhalla SL, et al. Prediction of the Oncotype DX recurrence score: use of pathology-generated equations derived by linear regression analysis. Mod Pathol. 2013; 26:658–64.
Article10. Kraus JA, Dabbs DJ, Beriwal S, Bhargava R. Semi-quantitative immunohistochemical assay versus oncotype DX((R)) qRT-PCR assay for estrogen and progesterone receptors: an independent quality assurance study. Mod Pathol. 2012; 25:869–76.11. Fitzgibbons PL, Dillon DA, Alsabeh R, Berman MA, Hayes DF, Hicks DG, et al. Template for reporting results of biomarker testing of specimens from patients with carcinoma of the breast. Arch Pathol Lab Med. 2014; 138:595–601.
Article12. Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007; 25:5287–312.
Article13. Vataire AL, Laas E, Aballea S, Gligorov J, Rouzier R, Chereau E. Cost-effectiveness of a chemotherapy predictive test. Bull Cancer. 2012; 99:907–14.14. Blohmer JU, Rezai M, Kummel S, Kuhn T, Warm M, Friedrichs K, et al. Using the 21-gene assay to guide adjuvant chemotherapy decision-making in early-stage breast cancer: a cost-effectiveness evaluation in the German setting. J Med Econ. 2013; 16:30–40.
Article15. Orucevic A, Heidel RE, Bell JL. Utilization and impact of 21-gene recurrence score assay for breast cancer in clinical practice across the United States: lessons learned from the 2010 to 2012 National Cancer Data Base analysis. Breast Cancer Res Treat. 2016; 157:427–35.
Article16. Albanell J, Svedman C, Gligorov J, Holt SD, Bertelli G, Blohmer JU, et al. Pooled analysis of prospective European studies assessing the impact of using the 21-gene Recurrence Score assay on clinical decision making in women with oestrogen receptor-positive, human epidermal growth factor receptor 2-negative early-stage breast cancer. Eur J Cancer. 2016; 66:104–13.
Article17. Carlson JJ, Roth JA. The impact of the Oncotype Dx breast cancer assay in clinical practice: a systematic review and meta-analysis. Breast Cancer Res Treat. 2013; 141:13–22.
Article18. Clark BZ, Dabbs DJ, Cooper KL, Bhargava R. Impact of progesterone receptor semiquantitative immunohistochemical result on Oncotype DX recurrence score: a quality assurance study of 1074 cases. Appl Immunohistochem Mol Morphol. 2013; 21:287–91.19. Zbytek B, Cohen C, Wang J, Page A, Williams DJ, Adams AL. Nottingham-defined mitotic score: comparison with visual and image cytometric phosphohistone H3 labeling indices and correlation with Oncotype DX recurrence score. Appl Immunohistochem Mol Morphol. 2013; 21:48–53.20. Cuzick J, Dowsett M, Pineda S, Wale C, Salter J, Quinn E, et al. Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the Genomic Health recurrence score in early breast cancer. J Clin Oncol. 2011; 29:4273–8.
Article21. Chen LL, Nolan ME, Silverstein MJ, Mihm MC Jr, Sober AJ, Tanabe KK, et al. The impact of primary tumor size, lymph node status, and other prognostic factors on the risk of cancer death. Cancer. 2009; 115:5071–83.
Article22. Orucevic A, Bell JL, McNabb AP, Heidel RE. Oncotype DX breast cancer recurrence score can be predicted with a novel nomogram using clinicopathologic data. Breast Cancer Res Treat. 2017; 163:51–61.
Article23. Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018; 379:111–21.
Article24. Williams DJ, Cohen C, Darrow M, Page AJ, Chastain B, Adams AL. Proliferation (Ki-67 and phosphohistone H3) and Oncotype DX recurrence score in estrogen receptor-positive breast cancer. Appl Immunohistochem Mol Morphol. 2011; 19:431–6.
Article25. Sahebjam S, Aloyz R, Pilavdzic D, Brisson ML, Ferrario C, Bouganim N, et al. Ki 67 is a major, but not the sole determinant of Oncotype Dx recurrence score. Br J Cancer. 2011; 105:1342–5.
Article26. Tang P, Wang J, Hicks DG, Wang X, Schiffhauer L, McMahon L, et al. A lower Allred score for progesterone receptor is strongly associated with a higher recurrence score of 21-gene assay in breast cancer. Cancer Invest. 2010; 28:978–82.
Article27. Auerbach J, Kim M, Fineberg S. Can features evaluated in the routine pathologic assessment of lymph node-negative estrogen receptor-positive stage I or II invasive breast cancer be used to predict the Oncotype DX recurrence score? Arch Pathol Lab Med. 2010; 134:1697–701.
Article28. Chaudhary LN, Jawa Z, Szabo A, Visotcky A, Chitambar CR. Relevance of progesterone receptor immunohistochemical staining to Oncotype DX recurrence score. Hematol Oncol Stem Cell Ther. 2016; 9:48–54.
Article29. Gage MM, Rosman M, Mylander WC, Giblin E, Kim HS, Cope L, et al. A validated model for identifying patients unlikely to benefit from the 21-gene recurrence score assay. Clin Breast Cancer. 2015; 15:467–72.30. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ, et al. Strategies for subtypes: dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011; 22:1736–47.31. Sparano JA, Paik S. Development of the 21-gene assay and its application in clinical practice and clinical trials. J Clin Oncol. 2008; 26:721–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prediction of Oncotype DX Recurrence Score Using Clinicopathological Variables in Estrogen Receptor-Positive/ Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer
- A simplified risk scoring system for predicting high-risk groups in gene expression tests for patients with estrogen receptor-positive, human epidermal growth factor receptor 2-negative, and node-positive breast cancer
- Diagnosis and Treatment of HER2-Positive Breast Cancer
- Prediction of Oncotype DX Recurrence Score Based on Systematic Evaluation of Ki-67 Scores in Hormone ReceptorPositive Early Breast Cancer
- Impact of Oncotype DX Recurrence Score on the Patterns of Locoregional Recurrence in Breast Cancer (Korean Radiation Oncology Group 19-06)