Yonsei Med J.
2012 Sep;53(5):1028-1035.
Development of the Rectus Abdominis and Its Sheath in the Human Fetus
- Affiliations
-
- 1Department of Surgery, Chonbuk National University Medical School, Jeonju, Korea. chobh@jbnu.ac.kr
- 2Department of Anatomy, Chonbuk National University Medical School, Jeonju, Korea.
- 3Department of Anatomy and Embryology II, Faculty of Medicine, Complutense University, Madrid, Spain.
- 4Oral Health Science Center hrc-8 and Department of Anatomy, Tokyo Dental College, Chiba, Japan.
- 5Division of Internal Medicine, Iwamizawa Kojin-kai Hospital, Iwamizawa, Japan.
- 6Research Institute of Clinical Medicine, Chonbuk National University Hospital, Jeonju, Korea.
Abstract
- PURPOSE
Although the rectus abdominis and its sheath are well known structures, their development in the human fetus is poorly understood.
MATERIALS AND METHODS
We examined rectus abdominis and sheath development in semiserial horizontal sections of 18 fetuses at 5-9 weeks of gestation.
RESULTS
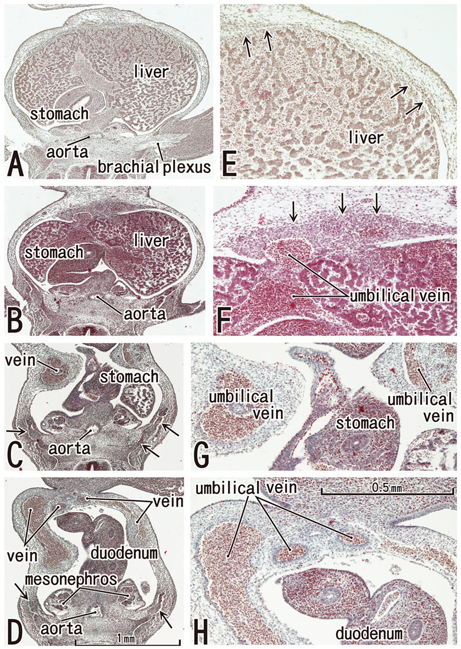
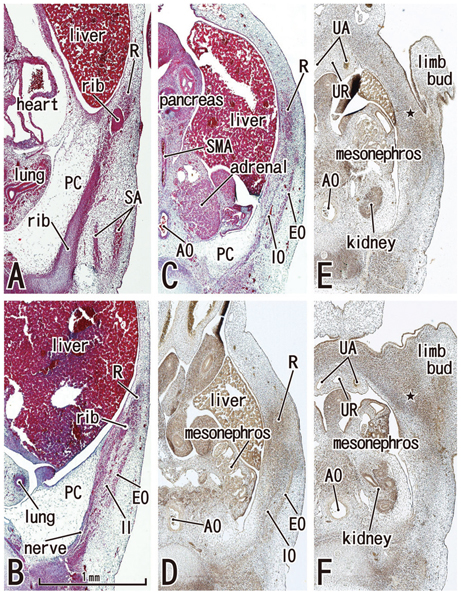
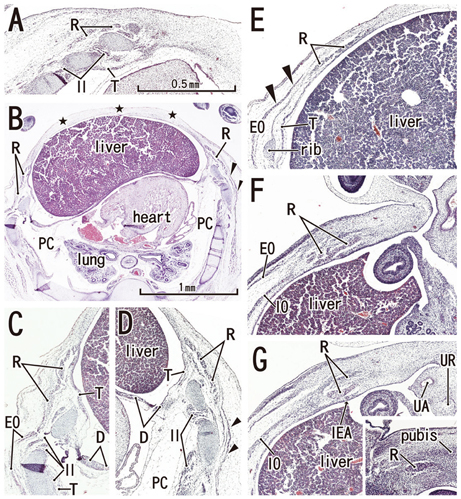
Rectus muscle differentiation was found to commence above the umbilicus at 6 weeks and extend inferiorly. Until closure of the anterior chest wall via fusion of the bilateral sternal anlagen (at 7 weeks), the anterior rectal sheath originated from the external oblique and developed towards the medial margin of the rectus abdominis at all levels, including the supracostal part. After formation of the anterior sheath, fascial laminae from the internal oblique and transversus abdominis contributed to formation of the posterior rectus sheath. However, the posterior sheath was absent along the supracostal part of the rectus abdominis, as the transversus muscle fibers reached the sternum or the midline area. Therefore, it appeared that resolution of the physiological umbilical hernia (8-9 weeks) as well as chest wall closure was not required for development of the rectus abdominis and its sheath. Conversely, in the inferior part of the two largest fetal specimens, after resolution of the hernia, the posterior sheath underwent secondary disappearance, possibly due to changes in mechanical stress.
CONCLUSION
Upward extension of the rectus abdominis suddenly stopped at the margin of the inferiorly developing pectoralis major without facing the external intercostalis. The rectus thoracis, if present, might correspond to the pectoralis.
Keyword
MeSH Terms
Figure
Reference
-
1. Williams PL. Gray's Anatomy. 1995. 38th ed. Edinburgh: Churchill Livingstone;825–829.2. Munger GT, Munger BL. Differentiation of the anterior body wall and truncal epidermis and associated co-migration of cutaneous nerves and mesenchyme. Anat Rec. 1991. 231:261–274.
Article3. Gościcka D, Murawski E. Tendinous intersections of the rectus abdominis muscle in human fetuses. Folia Morphol (Warsz). 1980. 39:427–434.4. Niikura H, Okamoto S, Nagase S, Takano T, Murakami G, Tatsumi H, et al. Fetal development of the human gubernaculum with special reference to the fasciae and muscles around it. Clin Anat. 2008. 21:547–557.
Article5. Rizk NN. A new description of the anterior abdominal wall. Anat Rec. 1976. 184:515.6. Rizk NN. A new description of the anterior abdominal wall in man and mammals. J Anat. 1980. 131(Pt 3):373–385.7. Rizk NN, Adieb N. The development of the anterior abdominal wall in the rat in the light of a new anatomical description. J Anat. 1982. 134(Pt 2):237–242.8. Rao VS, Rao GRKH. The sternalis muscle. J Anat Soc India. 1954. 3:49–51.9. Kacker GN. Sternalis muscle in U.P. Indian subjects. J Anat Soc India. 1960. 9:101–103.10. Blees G. A peculiar type of sternalis muscle. Acta Morphol Neerl Scand. 1968. 7:69–72.11. Jeng H, Su SJ. The sternalis muscle: an uncommon anatomical variant among Taiwanese. J Anat. 1998. 193(Pt 2):287–288.
Article12. Saeed M, Murshid KR, Rufai AA, Elsayed SE, Sadiq MS. Sternalis. An anatomic variant of chest wall musculature. Saudi Med J. 2002. 23:1214–1221.13. Rozen WM, Kapila S, Donahoe S. Why there are two rows of deep inferior epigastric artery perforators despite variability in the number of deep inferior epigastric artery trunks: an anatomical and embryological argument. Clin Anat. 2011. 24:786–788.
Article14. Klíma M. Early development of the human sternum and the problem of homologization of the so-called suprasternal structures. Acta Anat (Basel). 1968. 69:473–484.
Article15. Manley NR, Barrow JR, Zhang T, Capecchi MR. Hoxb2 and hoxb4 act together to specify ventral body wall formation. Dev Biol. 2001. 237:130–144.
Article16. Brewer S, Williams T. Loss of AP-2alpha impacts multiple aspects of ventral body wall development and closure. Dev Biol. 2004. 267:399–417.
Article17. Vermeij-Keers C, Hartwig NG, van der Werff JF. Embryonic development of the ventral body wall and its congenital malformations. Semin Pediatr Surg. 1996. 5:82–89.18. Stevenson RE, Rogers RC, Chandler JC, Gauderer MW, Hunter AG. Escape of the yolk sac: a hypothesis to explain the embryogenesis of gastroschisis. Clin Genet. 2009. 75:326–333.
Article19. Christ B, Jacob M, Jacob HJ. On the origin and development of the ventrolateral abdominal muscles in the avian embryo. An experimental and ultrastructural study. Anat Embryol (Berl). 1983. 166:87–101.
Article20. Durland JL, Sferlazzo M, Logan M, Burke AC. Visualizing the lateral somitic frontier in the Prx1Cre transgenic mouse. J Anat. 2008. 212:590–602.
Article21. Nowicki JL, Takimoto R, Burke AC. The lateral somitic frontier: dorso-ventral aspects of anterio-posterior regionalization in avian embryos. Mech Dev. 2003. 120:227–240.
Article22. Liem IK, Aoyama H. Body wall morphogenesis: limb-genesis interferes with body wall-genesis via its influence on the abaxial somite derivatives. Mech Dev. 2009. 126:198–211.
Article23. Evans DJ, Valasek P, Schmidt C, Patel K. Skeletal muscle translocation in vertebrates. Anat Embryol (Berl). 2006. 211:Suppl 1. 43–50.
Article24. Mehta V, Arora J, Yadav Y, Suri RK, Rath G. Rectus thoracis bifurcalis: a new variant in the anterior chest wall musculature. Rom J Morphol Embryol. 2010. 51:799–801.25. Ura R. Über die allgemeine Differenzierung der oberflächlichen Brustmuskeln mit besonderer Berűcksichtigung der Hautrumpfmuskeln der Säugetiere. Mitteil Med Gesellsch Tokyo. 1937. 51:216–288. 339–390.26. Kida MY, Izumi A, Tanaka S. Sternalis muscle: topic for debate. Clin Anat. 2000. 13:138–140.
Article27. O'Neill MN, Folan-Curran J. Case report: bilateral sternalis muscles with a bilateral pectoralis major anomaly. J Anat. 1998. 193(Pt 2):289–292.28. Jelev L, Georgiev G, Surchev L. The sternalis muscle in the Bulgarian population: classification of sternales. J Anat. 2001. 199(Pt 3):359–363.
Article29. Kitamura S, Yoshioka T, Kaneda M, Matsuoka K, Chen KL, Sakai A. [A case of the congenital partial defect of the pectoralis major--accompanied by the sternalis with enormous size]. Kaibogaku Zasshi. 1985. 60:728–732.30. Kida MY, Kudoh H. Innervation of the sternalis muscle accompanied by congenital partial absence of the pectoralis major muscle. Okajimas Folia Anat Jpn. 1991. 67:449–455.
Article31. Kumar H, Rath G, Sharma M, Kohli M, Rani B. Bilateral sternalis with unusual left-sided presentation: a clinical perspective. Yonsei Med J. 2003. 44:719–722.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Synchronous Development of Schwannoma in the Rectus Abdominis and Lipoma in the Chest: A Case Report
- Surgical anatomy of transversus abdominis muscle for transversus abdominis release
- Absence of Linea Alba in Breast Reconstruction with Pedicled TRAM Flap: A Case Report
- Rectus abdominis muscle atrophy after thoracotomy
- Embolization of Inferior Epigastric Artery for Treatment of Rectus Sheath Hematoma Caused by Insulin Injection During Anticoagulation and Antiplatelet Therapy