Anat Cell Biol.
2019 Jun;52(2):176-182. 10.5115/acb.2019.52.2.176.
The effect of alpha-lipoic acid on expression of VCAM-1 in type 2 diabetic rat
- Affiliations
-
- 1Department of Biochemistry, Faculty of Medicine, Riau University, Pekanbaru, Indonesia. ismawati75@yahoo.com
- 2Department of Internal Medicine, Faculty of Medicine, Riau University, Pekanbaru, Indonesia.
- 3Department of Pathology Anatomy, Faculty of Medicine, Riau University, Pekanbaru, Indonesia.
- KMID: 2451221
- DOI: http://doi.org/10.5115/acb.2019.52.2.176
Abstract
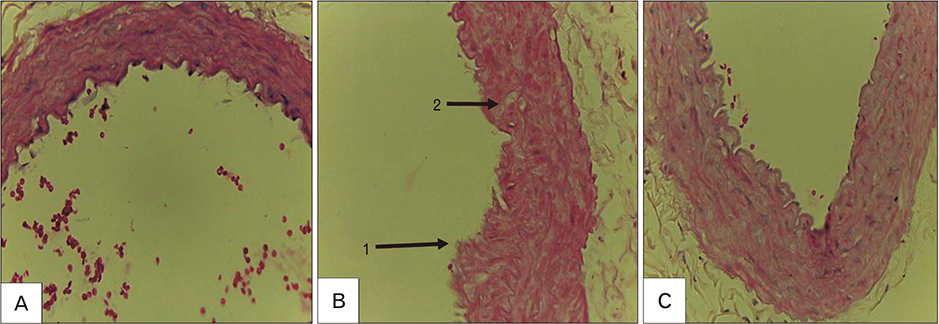
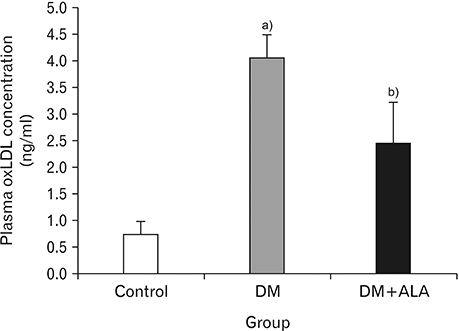
- Macrovascular diabetes complications are generally caused by a process called atherosclerosis. Evidences suggest that to initiate atherosclerosis, oxidated low-density lipoprotein (oxLDL) has to promote the expression of adhesion molecule. Several studies have evidenced the relevance of oxidative stress and atherosclerosis. However, the protective effect of alpha-lipoic acid (ALA) at atherosclerosis still needs to be explored. This study is aimed at investigating the concentration of plasma oxLDL and the expression of adhesion molecule of type 2 diabetes mellitus (DM) using rat model. Eighteen male rats were segregated into three groups labeled as control group, DM group and DM+ALA group. Type 2 diabetes was induced by intraperitoneal injection of streptozotocin (50 mg/kg) followed by nicotinamide (110 mg/kg). ALA was administered at a dose of 60 mg/kg body weight/day throughout the feeding period of 3 weeks. Plasma oxLDL concentration was measured by enzyme-linked immunosorbent assays and expression of vascular cell adhesion molecule-1 (VCAM-1) was measured by immunohistochemistry. Expression of abdominal aortic adhesion molecule was assessed by calculation with Adobe Photoshop CS3. Analysis of variance test was used to compare the concentration of plasma oxLDL and expression of adhesion molecule. A P-value of 0.05 was considered statistically significant. Plasma oxLDL was lower in diabetic rat+ALA compared with the diabetic rat. Percentage of area VCAM-1 in DM+ALA group was lower than DM group. There were no significant differences between groups in intensity of VCAM-1. In conclusion, ALA showed protective effects against early atherosclerosis in diabetic rats.
MeSH Terms
-
Animals
Atherosclerosis
Diabetes Complications
Diabetes Mellitus
Diabetes Mellitus, Type 2
Enzyme-Linked Immunosorbent Assay
Humans
Immunohistochemistry
Injections, Intraperitoneal
Lipoproteins
Male
Models, Animal
Niacinamide
Oxidative Stress
Plasma
Rats*
Streptozocin
Thioctic Acid*
Vascular Cell Adhesion Molecule-1*
Lipoproteins
Niacinamide
Streptozocin
Thioctic Acid
Vascular Cell Adhesion Molecule-1
Figure
Cited by 1 articles
-
Diabetes disrupts osteometric and trabecular morphometric parameters in the Zucker Diabetic Sprague–Dawley rat femur
Robert Ndou, Vaughan Perry, Gcwalisile Frances Dlamini
Anat Cell Biol. 2024;57(2):294-304. doi: 10.5115/acb.24.008.
Reference
-
1. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014; 37:Suppl 1. S81–S90.2. Oh YS. Mechanistic insights into pancreatic beta-cell mass regulation by glucose and free fatty acids. Anat Cell Biol. 2015; 48:16–24.
Article3. Gorąca A, Huk-Kolega H, Piechota A, Kleniewska P, Ciejka E, Skibska B. Lipoic acid: biological activity and therapeutic potential. Pharmacol Rep. 2011; 63:849–858.4. Abdullah ZA. Analisa faktor risiko diabetes melitus tipe 2 di Puskesmas Tanrutedong, Sidenreng Rappang. Medika. 2009; 4:228–229.5. Manrique CM, Rosenzweig JL, Umpiérrez GE. Diabetes, dyslipidemia, and heart protection [Internet]. The Hormone Foundation;2009. cited 2018 Dec 10. Available from: http://annarborendo.com/docs/diabetes-dyslipidemia-and-heartbilingual-071309.pdf.6. Mizuno Y, Jacob RF, Mason RP. Inflammation and the development of atherosclerosis. J Atheroscler Thromb. 2011; 18:351–358.
Article7. Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol. 1995; 15:1512–1531.8. Wu JT, Wu LL. Association of soluble markers with various stages and major events of atherosclerosis. Ann Clin Lab Sci. 2005; 35:240–250.9. Dunn S, Vohra RS, Murphy JE, Homer-Vanniasinkam S, Walker JH, Ponnambalam S. The lectin-like oxidized low-density-lipoprotein receptor: a pro-inflammatory factor in vascular disease. Biochem J. 2008; 409:349–355.
Article10. Galkina E, Ley K. Vascular adhesion molecules in atherosclerosis. Arterioscler Thromb Vasc Biol. 2007; 27:2292–2301.
Article11. Herrmann J, Lerman LO, Lerman A. On to the road to degradation: atherosclerosis and the proteasome. Cardiovasc Res. 2010; 85:291–302.
Article12. Stocker R, Keaney JF Jr. Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004; 84:1381–1478.
Article13. Islam MT. Antioxidant activities of dithiol alpha-lipoic acid. Bangladesh J Med Sci. 2009; 8:46–51.
Article14. Yi X, Maeda N. Alpha-lipoic acid prevents the increase in atherosclerosis induced by diabetes in apolipoprotein E-deficient mice fed high-fat/low-cholesterol diet. Diabetes. 2006; 55:2238–2244.15. Chaudhary P, Marracci GH, Bourdette DN. Lipoic acid inhibits expression of ICAM-1 and VCAM-1 by CNS endothelial cells and T cell migration into the spinal cord in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2006; 175:87–96.
Article16. Jiang S, Zhu W, Li C, Zhang X, Lu T, Ding Z, Cao K, Liu L. Alpha-lipoic acid attenuates LPS-induced cardiac dysfunction through a PI3K/Akt-dependent mechanism. Int Immunopharmacol. 2013; 16:100–107.17. Kunt T, Forst T, Wilhelm A, Tritschler H, Pfuetzner A, Harzer O, Engelbach M, Zschaebitz A, Stofft E, Beyer J. Alpha-lipoic acid reduces expression of vascular cell adhesion molecule-1 and endothelial adhesion of human monocytes after stimulation with advanced glycation end products. Clin Sci (Lond). 1999; 96:75–82.18. Annadurai T, Muralidharan AR, Joseph T, Hsu MJ, Thomas PA, Geraldine P. Antihyperglycemic and antioxidant effects of a flavanone, naringenin, in streptozotocin-nicotinamide-induced experimental diabetic rats. J Physiol Biochem. 2012; 68:307–318.
Article19. Ismawati , Winarto , Sari RP. Pencegahan lesi aterosklerosis oleh asam alfa lipoat pada aorta mencit jantan (Mus musculus) yang diberi diet tinggi kolesterol. J Ilmu Kedokteran. 2011; 4:19–25.20. Ismawati , Oenzil F, Yanwirasti , Yerizel E. Changes in expression of proteasome in rats at different stages of atherosclerosis. Anat Cell Biol. 2016; 49:99–106.
Article21. Balkis Budin S, Othman F, Louis SR, Abu Bakar M, Radzi M, Osman K, Das S, Mohamed J. Effect of alpha lipoic acid on oxidative stress and vascular wall of diabetic rats. Rom J Morphol Embryol. 2009; 50:23–30.22. Golbidi S, Badran M, Laher I. Diabetes and alpha lipoic acid. Front Pharmacol. 2011; 2:69.
Article23. Sena CM, Nunes E, Louro T, Proenca T, Fernandes R, Boarder MR, Seiça RM. Effects of alpha-lipoic acid on endothelial function in aged diabetic and high-fat fed rats. Br J Pharmacol. 2008; 153:894–906.24. Heinisch BB, Francesconi M, Mittermayer F, Schaller G, Gouya G, Wolzt M, Pleiner J. Alpha-lipoic acid improves vascular endothelial function in patients with type 2 diabetes: a placebo-controlled randomized trial. Eur J Clin Invest. 2010; 40:148–154.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Suppression of VEGF and STAT3 by Lipoic acid in Experimental Diabetic Rat Retina
- Effect of 12-week Oral Treatment with alpha-lipoic acid on the Nerve Conduction in Symptomatic Diabetic Neuropathy
- Efficacy and Safety of α-Lipoic Acid and Low Dose Pregabalin Combination in Painful Diabetic Neuropathy
- The Effect of alpha-Lipoic Acid on Vascular Smooth Muscle Cell Proliferation, Migration, Neointimal Formation and PAI-1 Expression
- Effects of alpha-lipoic acid on cell proliferation and apoptosis in MDA-MB-231 human breast cells