Tuberc Respir Dis.
2019 Jul;82(3):211-216. 10.4046/trd.2018.0019.
The Clinical Efficacy and Safety of Four-Weekly Docetaxel as First-Line Therapy in Elderly Lung Cancer Patients with Squamous Cell Carcinoma
- Affiliations
-
- 1Department of Internal Medicine, Korea University Guro Hospital, Seoul, Korea. syl0801@korea.ac.kr
- 2Department of Internal Medicine, Inje University Busan Paik Hospital, Busan, Korea.
- KMID: 2450898
- DOI: http://doi.org/10.4046/trd.2018.0019
Abstract
- BACKGROUND
Docetaxel is one of the standard treatments for advanced non-small cell lung cancer. Docetaxel is usually administered in a 3-week schedule, but there is significant toxicity. In this phase II clinical study, we investigated the efficacy and safety of a 4-weekly schedule of docetaxel monotherapy, as first-line chemotherapy for advanced squamous cell carcinoma in elderly lung cancer patients.
METHODS
Patients with stage IIIB/ IV lung squamous-cell carcinoma age 70 or older, that had not undergone cytotoxic chemotherapy were enrolled. Patients received docetaxel 25 mg/m2 on days 1, 8, and 15, every 4 weeks. Primary endpoint was the objective response rate (ORR). Secondary endpoints were progression-free survival (PFS), overall survival (OS), and toxicity profiles.
RESULTS
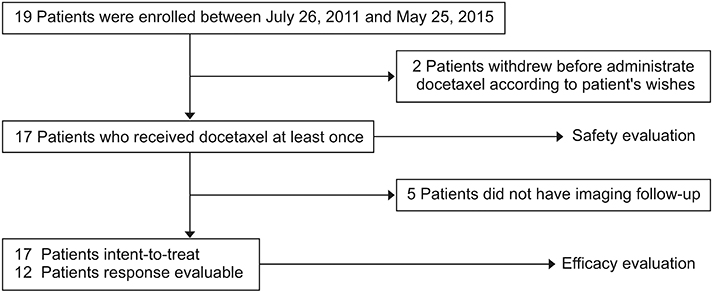
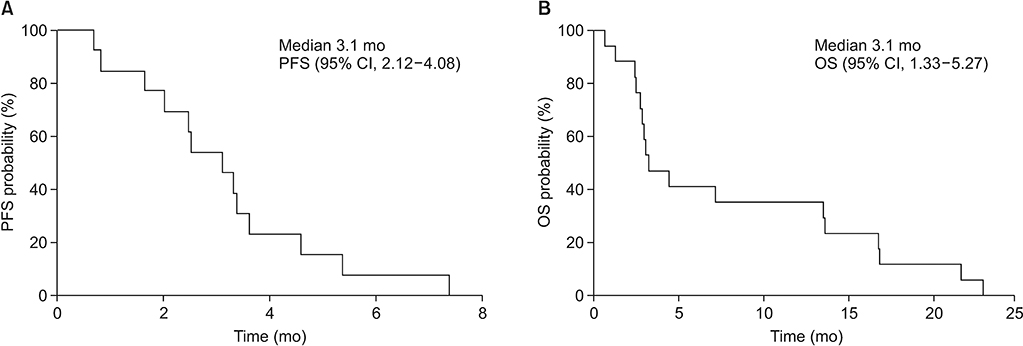
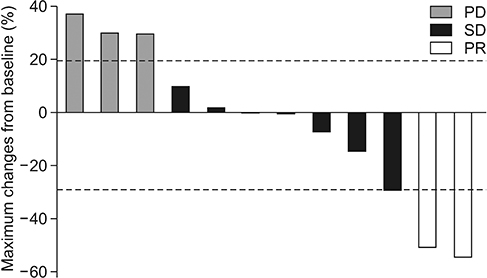
A total of 19 patients were enrolled. Among 19 patients, 17 were for evaluated efficacy and safety. In the intent-to-treat population, ORR and disease control rate (DCR) were 11.8% and 47.1%, respectively. In the response evaluable population, ORR was 16.7% and DCR was 66.7%. Median PFS and OS were 3.1 months and 3.3 months, respectively. There were three adverse grade 3/4 events. Grade 1 neutropenia was reported in one patient.
CONCLUSION
Our data failed to demonstrate efficacy of a 4-weekly docetaxel regimen, in elderly patients with a poor performance status. However, incidence of side effects, including neutropenia, was lower than with a 3-week docetaxel regimen, as previously reported.
Keyword
MeSH Terms
Figure
Reference
-
1. Livingston RB. Current management of unresectable non-small cell lung cancer. Semin Oncol. 1994; 21:5 Suppl 10. 4–11.2. Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002; 346:92–98.
Article3. Sekine I, Saijo N. Novel combination chemotherapy in the treatment of non-small cell lung cancer. Expert Opin Pharmacother. 2000; 1:1131–1161.
Article4. Clinical practice guidelines for the treatment of unresectable non-small-cell lung cancer. Adopted on May 16, 1997 by the American Society of Clinical Oncology. J Clin Oncol. 1997; 15:2996–3018.5. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015; 373:1627–1639.
Article6. Peters S, Gettinger S, Johnson ML, Janne PA, Garassino MC, Christoph D, et al. Phase II trial of atezolizumab as first-line or subsequent therapy for patients with programmed death-ligand 1-selected advanced non-small-cell lung cancer (BIRCH). J Clin Oncol. 2017; 35:2781–2789.
Article7. Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016; 375:1823–1833.
Article8. Fossella FV, DeVore R, Kerr RN, Crawford J, Natale RR, Dunphy F, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol. 2000; 18:2354–2362.9. Kudoh S, Takeda K, Nakagawa K, Takada M, Katakami N, Matsui K, et al. Phase III study of docetaxel compared with vinorelbine in elderly patients with advanced non-small-cell lung cancer: results of the West Japan Thoracic Oncology Group Trial (WJTOG 9904). J Clin Oncol. 2006; 24:3657–3663.
Article10. Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O'Rourke M, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000; 18:2095–2103.
Article11. Bansal P, Osman D, Gan GN, Simon GR, Boumber Y. Recent advances in targetable therapeutics in metastatic non-squamous NSCLC. Front Oncol. 2016; 6:112.
Article12. Kudo S, Hino M, Fujita A, Igarashi T, Arita K, Niitani H, et al. Late phase II clinical study of RP56976 (docetaxel) in patients with non-small cell lung cancer. Gan To Kagaku Ryoho. 1994; 21:2617–2623.13. Hainsworth JD, Burris HA 3rd, Litchy S, Morrissey LH, Barton JH, Bradof JE, et al. Weekly docetaxel in the treatment of elderly patients with advanced nonsmall cell lung carcinoma. A Minnie Pearl Cancer Research Network Phase II Trial. Cancer. 2000; 89:328–333.14. Gridelli C, Gallo C, Di Maio M, Barletta E, Illiano A, Maione P, et al. A randomised clinical trial of two docetaxel regimens (weekly vs 3 week) in the second-line treatment of non-small-cell lung cancer. The DISTAL 01 study. Br J Cancer. 2004; 91:1996–2004.
Article15. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–247.
Article16. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–E386.
Article17. Rossi A, Gridelli C. Chemotherapy of advanced non-small cell lung cancer in elderly patients. Ann Oncol. 2006; 17:Suppl 2. ii58–ii60.
Article18. Lilenbaum RC. Treatment of advanced non-small-cell lung cancer in special populations. Oncology (Williston Park). 2004; 18:1321–1325.19. Midha A, Dearden S, McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII). Am J Cancer Res. 2015; 5:2892–2911.20. Kim KS, Oh IJ, Ban HJ, Cho HJ, Kwon YS, Kim YI, et al. Comparison of docetaxel/cisplatin dosages of 75/60 and 60/60 mg/m(2) for the treatment of non-small cell lung cancer. Exp Ther Med. 2012; 4:317–322.
Article21. Du Z, Qing J, Ye H, Zhang Z, Lu J. Effects of weekly dose docetaxel monotherapy schedule for elderly patients with non-small cell lung cancer. Chinese-German J Clin Oncol. 2009; 8:9.
Article22. Lilenbaum R, Rubin M, Samuel J, Boros L, Chidiac T, Seigel L, et al. A randomized phase II trial of two schedules of docetaxel in elderly or poor performance status patients with advanced non-small cell lung cancer. J Thorac Oncol. 2007; 2:306–311.
Article23. Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst. 1999; 91:1616–1634.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Weekly Low-Dose Docetaxel for Salvage Chemotherapy in Pretreated Elderly or Poor Performance Status Patients with Non-small Cell Lung Cancer
- Treatment of Advanced and Metastatic Squamous Non-small Cell Lung Cancer
- Docetaxel as Second-line Monotherapy for Advanced Non-small Cell Lung Cancer
- Cell Death Induction Mechanism of Non-small Cell Lung Cancer Cell Line, NCI-H1703 by Docetaxel
- Docetaxel Monotherapy as Second-Line Treatment for Pretreated Advanced Non-Small Cell Lung Cancer Patients