Investig Clin Urol.
2019 Jul;60(4):244-250. 10.4111/icu.2019.60.4.244.
Effectiveness of three different luteinizing hormone-releasing hormone agonists in the chemical castration of patients with prostate cancer: Goserelin versus triptorelin versus leuprolide
- Affiliations
-
- 1Department of Urology, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, Anyang, Korea. js315@hallym.or.kr
- KMID: 2450541
- DOI: http://doi.org/10.4111/icu.2019.60.4.244
Abstract
- PURPOSE
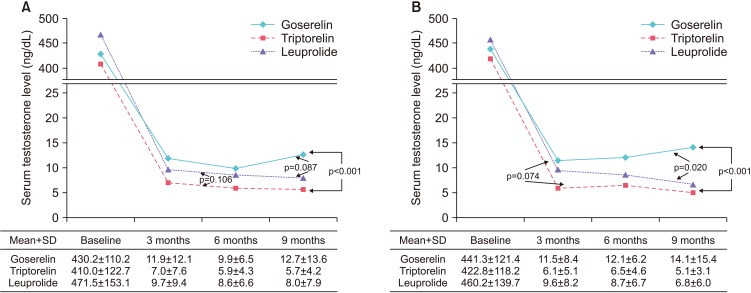
To investigate the changes in testosterone levels and rates of chemical castration following androgen-deprivation therapy (ADT) with goserelin, triptorelin, and leuprolide.
MATERIALS AND METHODS
We retrospectively reviewed the medical records of 125 patients with prostate cancer treated with luteinizing hormone-releasing hormone (LHRH) agonists between January 2009 and December 2015. Changes in testosterone concentration during 9 months of ADT with goserelin 11.34 mg, triptorelin 11.25 mg, and leuprolide 11.25 mg were analyzed using a mixed model. The number of patients with serum testosterone below castration levels defined as various values (<50 ng/dL, <20 ng/dL, or <10 ng/dL) at 3, 6, and 9 months were also evaluated.
RESULTS
Of the 125 patients, 59 received goserelin, 44 received triptorelin, and 22 received leuprolide, respectively. The lowest mean testosterone levels during 9 months of treatment were achieved in patients treated with triptorelin, followed by those treated with leuprolide, and then by those treated with goserelin (p=0.001). Significant differences in chemical castration levels were observed only at <10 ng/dL, with 54.2% of goserelin, 93.2% of triptorelin, and 86.4% of leuprolide treated patients (p<0.001).
CONCLUSIONS
Three LHRH agonists showed comparable efficacy for achieving castration when the castration threshold was 50 or 20 ng/dL. However, triptorelin was the most potent LHRH agonist, achieving the lowest mean testosterone levels and the highest rate of chemical castration at <10 ng/dL testosterone.
MeSH Terms
-
Antineoplastic Agents
Castration*
Gonadotropin-Releasing Hormone*
Goserelin*
Humans
Leuprolide*
Lutein*
Medical Records
Prostate*
Prostate-Specific Antigen
Prostatic Neoplasms*
Retrospective Studies
Testosterone
Triptorelin Pamoate*
Antineoplastic Agents
Gonadotropin-Releasing Hormone
Goserelin
Leuprolide
Lutein
Prostate-Specific Antigen
Testosterone
Triptorelin Pamoate
Figure
Reference
-
1. Denis L, Murphy GP. Overview of phase III trials on combined androgen treatment in patients with metastatic prostate cancer. Cancer. 1993; 72(12 Suppl):3888–3895. PMID: 8252511.
Article2. Seidenfeld J, Samson DJ, Hasselblad V, Aronson N, Albertsen PC, Bennett CL, et al. Single-therapy androgen suppression in men with advanced prostate cancer: a systematic review and meta-analysis. Ann Intern Med. 2000; 132:566–577. PMID: 10744594.3. Dias Silva É, Ferreira U, Matheus W, Faria EF, Silva GD, Saito M, et al. Goserelin versus leuprolide in the chemical castration of patients with prostate cancer. Int Urol Nephrol. 2012; 44:1039–1044. PMID: 22315155.
Article4. Schally AV, Arimura A, Kastin AJ, Matsuo H, Baba Y, Redding TW, et al. Gonadotropin-releasing hormone: one polypeptide regulates secretion of luteinizing and follicle-stimulating hormones. Science. 1971; 173:1036–1038. PMID: 4938639.
Article5. Kiesel L. Molecular mechanisms of gonadotrophin releasing hormone-stimulated gonadotrophin secretion. Hum Reprod. 1993; 8(Suppl 2):23–28. PMID: 8276964.6. Stojilkovic SS, Reinhart J, Catt KJ. Gonadotropin-releasing hormone receptors: structure and signal transduction pathways. Endocr Rev. 1994; 15:462–499. PMID: 7988482.
Article7. Heyns CF, Simonin MP, Grosgurin P, Schall R, Porchet HC. South African Triptorelin Study Group. Comparative efficacy of triptorelin pamoate and leuprolide acetate in men with advanced prostate cancer. BJU Int. 2003; 92:226–231. PMID: 12887472.
Article8. Yri OE, Bjoro T, Fossa SD. Failure to achieve castration levels in patients using leuprolide acetate in locally advanced prostate cancer. Eur Urol. 2006; 49:54–58. discussion 58. PMID: 16314038.
Article9. Ishitsuka R, Miyazaki J, Ichioka D, Inoue T, Kageyama S, Sugimoto M, et al. Impact of acute kidney injury defined by CTCAE v4.0 during first course of cisplatin-based chemotherapy on treatment outcomes in advanced urothelial cancer patients. Clin Exp Nephrol. 2017; 21:732–740. PMID: 27565169.
Article10. Oefelein MG, Feng A, Scolieri MJ, Ricchiutti D, Resnick MI. Reassessment of the definition of castrate levels of testosterone: implications for clinical decision making. Urology. 2000; 56:1021–1024. PMID: 11113751.
Article11. Breul J, Lundström E, Purcea D, Venetz WP, Cabri P, Dutailly P, et al. Efficacy of testosterone suppression with sustained-release triptorelin in advanced prostate cancer. Adv Ther. 2017; 34:513–523. PMID: 28028737.
Article12. Veldhuis JD, Liem AY, South S, Weltman A, Weltman J, Clemmons DA, et al. Differential impact of age, sex steroid hormones, and obesity on basal versus pulsatile growth hormone secretion in men as assessed in an ultrasensitive chemiluminescence assay. J Clin Endocrinol Metab. 1995; 80:3209–3222. PMID: 7593428.
Article13. Wheeler MJ, D'Souza A, Matadeen J, Croos P. Ciba Corning ACS:180 testosterone assay evaluated. Clin Chem. 1996; 42:1445–1449. PMID: 8787702.
Article14. Morote J, Orsola A, Planas J, Trilla E, Raventós CX, Cecchini L, et al. Redefining clinically significant castration levels in patients with prostate cancer receiving continuous androgen deprivation therapy. J Urol. 2007; 178:1290–1295. PMID: 17698136.
Article15. Dason S, Allard CB, Tong J, Shayegan B. Defining a new testosterone threshold for medical castration: results from a prospective cohort series. Can Urol Assoc J. 2013; 7:E263–E267. PMID: 23766827.
Article16. Kamada S, Sakamoto S, Ando K, Muroi A, Fuse M, Kawamura K, et al. Nadir testosterone after long-term followup predicts prognosis in patients with prostate cancer treated with combined androgen blockade. J Urol. 2015; 194:1264–1270. PMID: 25861958.
Article17. Klotz L, O'Callaghan C, Ding K, Toren P, Dearnaley D, Higano CS, et al. Nadir testosterone within first year of androgen-deprivation therapy (ADT) predicts for time to castration-resistant progression: a secondary analysis of the PR-7 trial of intermittent versus continuous ADT. J Clin Oncol. 2015; 33:1151–1156. PMID: 25732157.
Article18. Perachino M, Cavalli V, Bravi F. Testosterone levels in patients with metastatic prostate cancer treated with luteinizing hormone-releasing hormone therapy: prognostic significance? BJU Int. 2010; 105:648–651. PMID: 19747358.
Article19. Reis LO, Denardi F, Faria EF, Silva ED. Correlation between testosterone and PSA kinetics in metastatic prostate cancer patients treated with diverse chemical castrations. Am J Mens Health. 2015; 9:430–434. PMID: 25294865.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sterile Abscess Formation Associated with Two Different Forms of Gonadotropin-Releasing Hormone Agonist in Central Precocious Puberty
- Current Concepts in Androgen Deprivation Therapy
- Effect of GnRH Analogs Leuprolide-Acetate and Triptorelin on Bone Mineral Density in Girls with Central Precocious Puberty
- Durable Response of Androgen Receptor-Positive Male Breast Cancer to Goserelin
- Efficacy of Combined Aromatase Inhibitor and Luteinizing Hormone-Releasing Hormone Agonist in Premenopausal Metastatic Breast Cancer