J Pathol Transl Med.
2019 May;53(3):159-163. 10.4132/jptm.2019.03.14.
Primary Age-Related Tauopathy: An Elderly Brain Pathology Frequently Encountered during Autopsy
- Affiliations
-
- 1Department of Pathology, Chonnam National University Medical School, Hwasun, Korea. mdkaylee@jnu.ac.kr jhlee@chonnam.ac.kr
- 2Department of Forensic Medicine, Chonnam National University Medical School, Hwasun, Korea.
- 3Department of Neurology, Chonnam National University Medical School, Hwasun, Korea.
- KMID: 2449338
- DOI: http://doi.org/10.4132/jptm.2019.03.14
Abstract
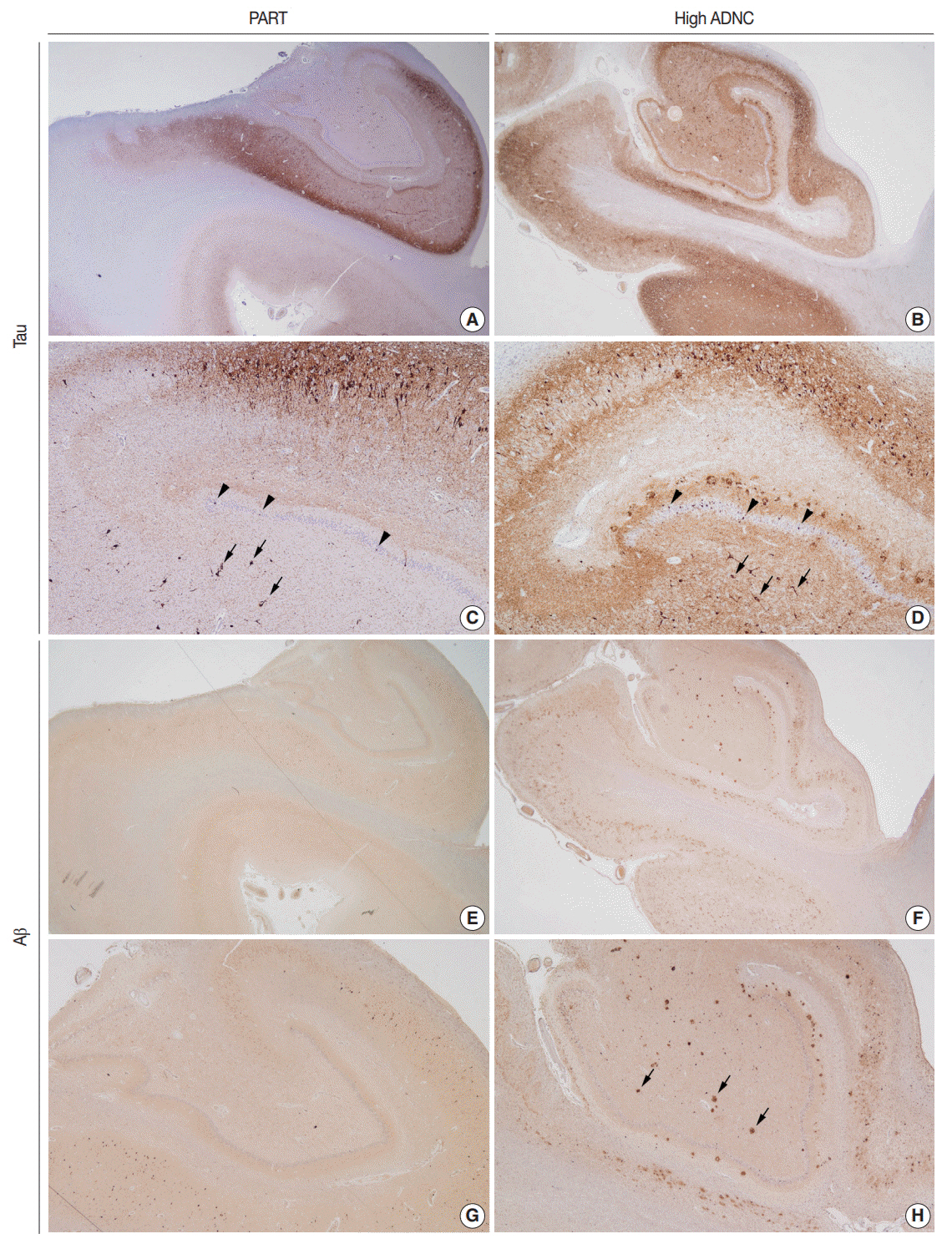
- Due to the progressive aging of Korean society and the introduction of brain banks to the Korean medical system, the possibility that pathologists will have access to healthy elderly brains has increased. The histopathological analysis of an elderly brain from a subject with relatively well-preserved cognition is quite different from that of a brain from a demented subject. Additionally, the histology of elderly brains differs from that of young brains. This brief review discusses primary age-related tauopathy; this term was coined to describe elderly brains with Alzheimer's diseasetype neurofibrillary tangles mainly confined to medial temporal structures, and no β-amyloid pathology.
Keyword
MeSH Terms
Figure
Reference
-
1. Crary JF, Trojanowski JQ, Schneider JA, et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 2014; 128:755–66.
Article2. Irwin DJ. Tauopathies as clinicopathological entities. Parkinsonism Relat Disord. 2016; 22 Suppl 1:S29–33.
Article3. Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012; 8:1–13.
Article4. Bennett RE, DeVos SL, Dujardin S, et al. Enhanced tau aggregation in the presence of amyloid beta. Am J Pathol. 2017; 187:1601–12.5. Crary JF. Primary age-related tauopathy and the amyloid cascade hypothesis: the exception that proves the rule? J Neurol Neuromedicine. 2016; 1:53–7.
Article6. Josephs KA, Murray ME, Tosakulwong N, et al. Tau aggregation influences cognition and hippocampal atrophy in the absence of beta-amyloid: a clinico-imaging-pathological study of primary agerelated tauopathy (PART). Acta Neuropathol. 2017; 133:705–15.
Article7. Kaufman SK, Del Tredici K, Thomas TL, Braak H, Diamond MI. Tau seeding activity begins in the transentorhinal/entorhinal regions and anticipates phospho-tau pathology in Alzheimer’s disease and PART. Acta Neuropathol. 2018; 136:57–67.
Article8. Neltner JH, Abner EL, Jicha GA, et al. Brain pathologies in extreme old age. Neurobiol Aging. 2016; 37:1–11.
Article9. Kovacs GG. Invited review: neuropathology of tauopathies: principles and practice. Neuropathol Appl Neurobiol. 2015; 41:3–23.
Article10. Braak H, Del Tredici K. The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol. 2011; 121:171–81.
Article11. Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011; 70:960–9.
Article12. Jicha GA, Parisi JE, Dickson DW, et al. Age and apoE associations with complex pathologic features in Alzheimer's disease. J Neurol Sci. 2008; 273:34–9.
Article13. Alzheimer’s Association. 2016 Alzheimer's disease facts and figures. Alzheimers Dement. 2016; 12:459–509.14. Janocko NJ, Brodersen KA, Soto-Ortolaza AI, et al. Neuropathologically defined subtypes of Alzheimer's disease differ significantly from neurofibrillary tangle-predominant dementia. Acta Neuropathol. 2012; 124:681–92.
Article15. Yamada M. Senile dementia of the neurofibrillary tangle type (tangle-only dementia): neuropathological criteria and clinical guidelines for diagnosis. Neuropathology. 2003; 23:311–7.
Article16. Jellinger KA, Attems J. Neurofibrillary tangle-predominant dementia: comparison with classical Alzheimer disease. Acta Neuropathol. 2007; 113:107–17.
Article17. Kryscio RJ, Abner EL, Jicha GA, et al. Self-reported memory complaints: a comparison of demented and unimpaired outcomes. J Prev Alzheimers Dis. 2016; 3:13–9.
Article18. Nelson PT, Trojanowski JQ, Abner EL, et al. “New old pathologies”: AD, PART, and cerebral age-related TDP-43 with sclerosis (CARTS). J Neuropathol Exp Neurol. 2016; 75:482–98.
Article19. Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW. Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol. 2011; 10:785–96.
Article20. Josephs KA, Whitwell JL, Tosakulwong N, et al. TAR DNA-binding protein 43 and pathological subtype of Alzheimer’s disease impact clinical features. Ann Neurol. 2015; 78:697–709.
Article21. Jack CR Jr. PART and SNAP. Acta Neuropathol. 2014; 128:773–6.
Article22. Duyckaerts C, Braak H, Brion JP, et al. PART is part of Alzheimer disease. Acta Neuropathol. 2015; 129:749–56.
Article23. Robinson JL, Corrada MM, Kovacs GG, et al. Non-Alzheimer’s contributions to dementia and cognitive resilience in The 90+ Study. Acta Neuropathol. 2018; 136:377–88.
Article