Chonnam Med J.
2019 May;55(2):109-115. 10.4068/cmj.2019.55.2.109.
Therapeutic Effect of Fimasartan in a Rat Model of Myocardial Infarction Evaluated by Cardiac Positron Emission Tomography with [¹â¸F]FPTP
- Affiliations
-
- 1Division of Cardiology, Chonnam National University Hospital, Gwangju, Korea. hyj200@hanmail.net jjmin@jnu.ac.kr
- 2Institute for Biomedical Science, Chonnam National University Hwasun Hospital, Hwasun, Korea.
- 3Department of Nuclear Medicine, Chonnam National University Hwasun Hospital, Hwasun, Korea. hyj200@hanmail.net jjmin@jnu.ac.kr
- 4Department of Nuclear Medicine, Seoul National University Hospital, Seoul, Korea.
- KMID: 2447213
- DOI: http://doi.org/10.4068/cmj.2019.55.2.109
Abstract
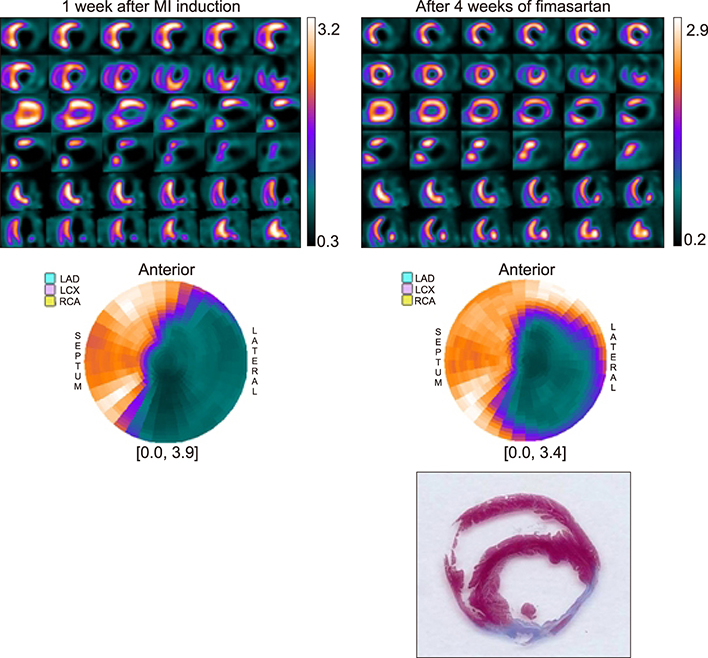
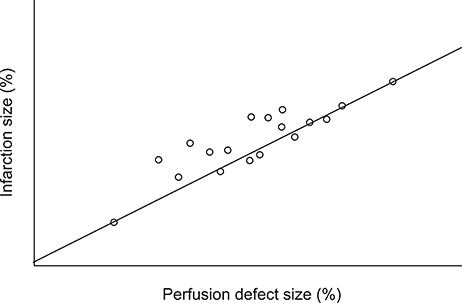
- We evaluated the efficacy of fimasartan on perfusion defects and infarction size in an animal model of myocardial infarction (MI), with echocardiography and positron emission tomography (PET) using a ¹â¸F-labeled phosphonium cation (5-[¹â¸F]-fluoropentyl-triphenylphosphonium salt, [¹â¸F]FPTP) as a mitochondrial voltage sensor for myocardial imaging. We induced MI in 33 rats by ligation of the left coronary artery, and checked their cardiac PET image using [¹â¸F]FPTP for evaluation of myocardial perfusion. Rats were grouped into 3 groups according to their administered drugs: no drug (n=11), fimasartan 3 mg/kg (n=10), and fimasartan 10 mg/kg (n=12). Each designated drug was administered for 4 weeks, and follow-up PET and histologic examinations were done. In the PET analysis, a perfusion defect size was markedly improved in fimasartan 10 mg/kg group (35.9±7.0% to 28.4±6.9%, p<0.001), whereas treatment with fimasartan 3 mg/kg induced only an insignificant reduction of perfusion defect size (35.9±7.9% to 33.9±7.3%, p=0.095). Using 2, 3, 5-triphenyltetrazolium chloride staining, infarction size was the largest in the control group (36.5±8.3%), and was insignificantly lower in the fimasartan 3 mg/kg group (31.5±6.5%, p for the difference between the control group=0.146) and was significantly lower in the fimasartan 10 mg/kg group (26.3±7.6%, p for the difference between the control group=0.011). PET imaging using a ¹â¸F-labeled mitochondrial voltage sensor, [¹â¸F]FPTP, is useful in evaluation and monitoring of myocardial perfusion states, and treatment with fimasartan decreases the infarction size in animal MI model.
MeSH Terms
Figure
Reference
-
1. Brundage BH, Massie BM, Botvinick EH. Improved regional ventricular function after successful surgical revascularization. J Am Coll Cardiol. 1984; 3:902–908.
Article2. Kim DY, Kim HS, Jang HY, Kim JH, Bom HS, Min JJ. Comparison of the cardiac microPET images obtained using [(18)F]FPTP and [(13)N]NH3 in rat myocardial infarction models. ACS Med Chem Lett. 2014; 5:1124–1128.
Article3. Yoshinaga K, Chow BJ, Williams K, Chen L, deKemp RA, Garrard L, et al. What is the prognostic value of myocardial perfusion imaging using rubidium-82 positron emission tomography? J Am Coll Cardiol. 2006; 48:1029–1039.
Article4. Kim DY, Kim HS, Le UN, Jiang SN, Kim HJ, Lee KC, et al. Evaluation of a mitochondrial voltage sensor, (18F-fluoropentyl) triphenylphosphonium cation, in a rat myocardial infarction model. J Nucl Med. 2012; 53:1779–1785.5. Kim DY, Kim HS, Reder S, Zheng JH, Herz M, Higuchi T, et al. Comparison of 18F-labeled fluoroalkylphosphonium cations with 13N-NH3 for PET myocardial perfusion imaging. J Nucl Med. 2015; 56:1581–1586.
Article6. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. ESC Scientific Document Group. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016; 37:2129–2200.
Article7. Kim JH, Lee JH, Paik SH, Kim JH, Chi YH. Fimasartan, a novel angiotensin II receptor antagonist. Arch Pharm Res. 2012; 35:1123–1126.
Article8. Lee JH, Yang DH, Hwang JY, Hur SH, Cha TJ, Kim KS, et al. A randomized, double-blind, candesartan-controlled, parallel group comparison clinical trial to evaluate the antihypertensive efficacy and safety of fimasartan in patients with mild to moderate essential hypertension. Clin Ther. 2016; 38:1485–1497.
Article9. Chang SA, Lim BK, Lee YJ, Hong MK, Choi JO, Jeon ES. A novel angiotensin type I receptor antagonist, fimasartan, prevents doxorubicin-induced cardiotoxicity in rats. J Korean Med Sci. 2015; 30:559–568.
Article10. Sim DS, Jeong MH, Song HC, Kim J, Chong A, Bom HS, et al. Cardioprotective effect of fimasartan, a new angiotensin receptor blocker, in a porcine model of acute myocardial infarction. J Korean Med Sci. 2015; 30:34–43.
Article11. Han J, Park SJ, Thu VT, Lee SR, Long le T, Kim HK, et al. Effects of the novel angiotensin II receptor type I antagonist, fimasartan on myocardial ischemia/reperfusion injury. Int J Cardiol. 2013; 168:2851–2859.
Article12. Siegrist PT, Husmann L, Knabenhans M, Gaemperli O, Valenta I, Hoefflinghaus T, et al. (13)N-ammonia myocardial perfusion imaging with a PET/CT scanner: impact on clinical decision making and cost-effectiveness. Eur J Nucl Med Mol Imaging. 2008; 35:889–895.
Article13. Sampson UK, Dorbala S, Limaye A, Kwong R, Di Carli MF. Diagnostic accuracy of rubidium-82 myocardial perfusion imaging with hybrid positron emission tomography/computed tomography in the detection of coronary artery disease. J Am Coll Cardiol. 2007; 49:1052–1058.
Article14. Chen LB. Mitochondrial membrane potential in living cells. Annu Rev Cell Biol. 1988; 4:155–181.
Article15. Toh N, Ishii K, Kihara H, Iwakura K, Watanabe H, Yoshikawa J, et al. Effect of ARB/diuretics on diastolic function in patients with hypertension 2 (EDEN2) trial investigators. Effect of diuretic or calcium-channel blocker plus angiotensin-receptor blocker on diastolic function in hypertensive patients. Circ J. 2016; 80:426–434.
Article16. Kario K, Tomitani N, Kanegae H, Ishii H, Uchiyama K, Yamagiwa K, et al. Comparative effects of an angiotensin II receptor blocker (ARB)/diuretic vs. ARB/calcium-channel blocker combination on uncontrolled nocturnal hypertension evaluated by information and communication technology-based nocturnal home blood pressure monitoring - the NOCTURNE study. Circ J. 2017; 81:948–957.
Article17. Miura M, Sakata Y, Miyata S, Shiba N, Takahashi J, Nochioka K, et al. SUPPORT Trial Investigators. Influence of left ventricular ejection fraction on the effects of supplemental use of angiotensin receptor blocker olmesartan in hypertensive patients with heart failure. Circ J. 2016; 80:2155–2164.
Article18. Park H, Kim HK, Jeong MH, Cho JY, Lee KH, Sim DS, et al. Clinical impacts of inhibition of renin-angiotensin system in patients with acute ST-segment elevation myocardial infarction who underwent successful late percutaneous coronary intervention. J Cardiol. 2017; 69:216–221.19. Lee SE, Kim YJ, Lee HY, Yang HM, Park CG, Kim JJ, et al. Investigators. Efficacy and tolerability of fimasartan, a new angiotensin receptor blocker, compared with losartan (50/100 mg): a 12-week, phase III, multicenter, prospective, randomized, double-blind, parallel-group, dose escalation clinical trial with an optional 12-week extension phase in adult Korean patients with mild-to-moderate hypertension. Clin Ther. 2012; 34:552–568. 568.e1–568.e9.
Article20. Park JB, Sung KC, Kang SM, Cho EJ. Safety and efficacy of fimasartan in patients with arterial hypertension (Safe-KanArb study): an open-label observational study. Am J Cardiovasc Drugs. 2013; 13:47–56.
Article21. Correa F, Soto V, Zazueta C. Mitochondrial permeability transition relevance for apoptotic triggering in the post-ischemic heart. Int J Biochem Cell Biol. 2007; 39:787–798.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Radiolabeled Phosphonium Salts as Mitochondrial Voltage Sensors for Positron Emission Tomography Myocardial Imaging Agents
- Emerging Tracers for Nuclear Cardiac PET Imaging
- Cardioprotective Effect of Fimasartan, a New Angiotensin Receptor Blocker, in a Porcine Model of Acute Myocardial Infarction
- Evaluation of Myocardial Blood Flow and Coronary Flow Reserve Using Positron Emission Tomography
- Recent Advances in Nuclear Cardiology