Chonnam Med J.
2019 May;55(2):75-85. 10.4068/cmj.2019.55.2.75.
Therapeutic Effects of Synthetic Antimicrobial Peptides, TRAIL and NRP1 Blocking Peptides in Psoriatic Keratinocytes
- Affiliations
-
- 1Department of Dermatology, University of Colorado Denver School of Medicine, Aurora, CO, USA. peter.song@ucdenver.edu
- 2Department of Biomedical Science and Research Center for Proteinaceous Materials, Chosun University School of Medicine, Gwangju, Korea.
- 3Department of Biology, University of Denver, Denver, CO, USA.
- 4Department of Dermatology, Chung-Ang University School of Medicine, Seoul, Korea.
- KMID: 2447209
- DOI: http://doi.org/10.4068/cmj.2019.55.2.75
Abstract
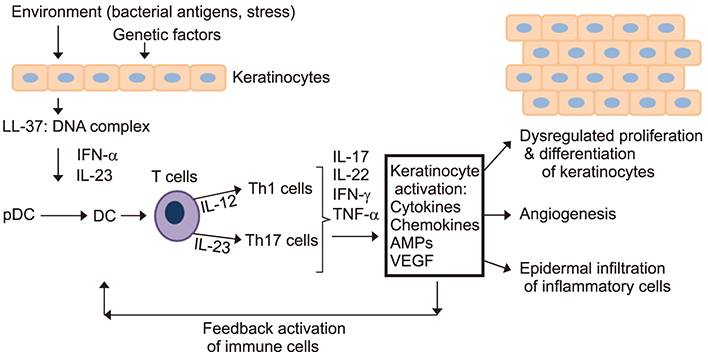
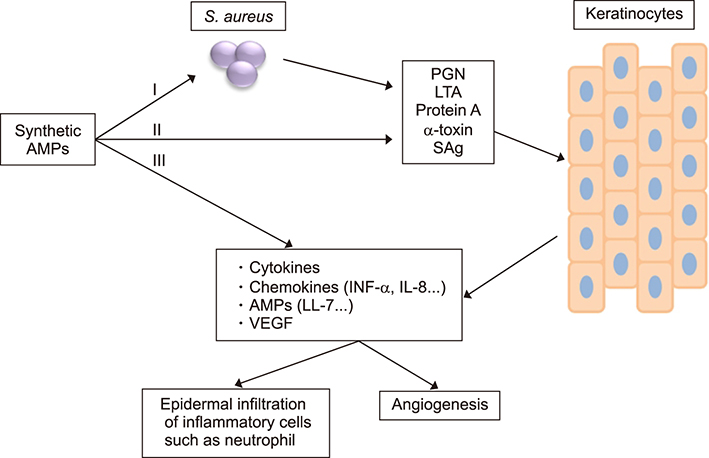
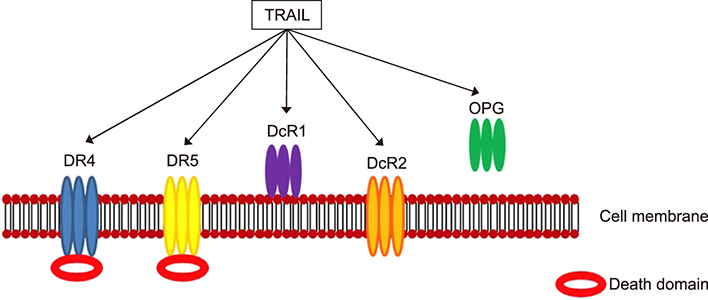
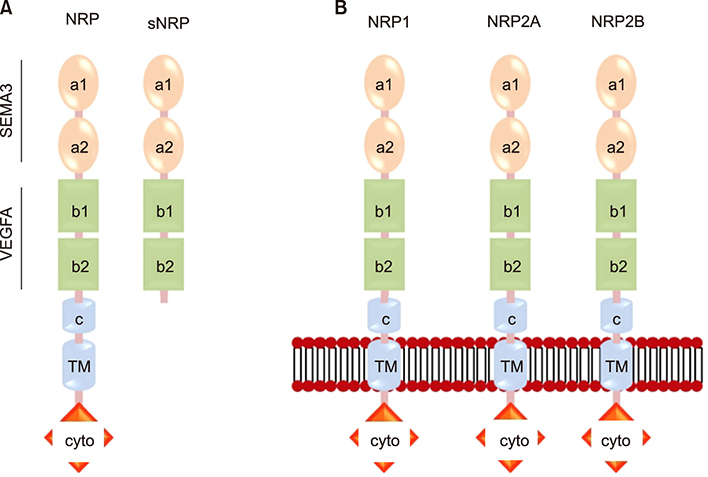
- Psoriasis is a chronic, recurrent, heterogeneous, cutaneous inflammatory skin disease for which there is no cure. It affects approximately 7.5 million people in the United States. Currently, several biologic agents that target different molecules implicated in the pathogenic processes of psoriasis are being assessed in diverse clinical studies. However, relapse usually occurs within weeks or months, meaning there is currently no cure for psoriasis. Therefore, recent studies have discovered diverse new potential treatments for psoriasis: inhibitors of bacteria such as Staphylococcus aureus, tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and neuropilin 1 (NRP1). A promising approach that has recently been described involves modifying antimicrobial peptides to develop new cutaneous anti-bacterial agents that target inflammatory skin disease induced by Staphylococcus. Increased expression of TRAIL and its death receptors DR4 and DR5 has been implicated in the pathogenesis of plaque psoriasis. In addition, TRAIL has the ability to inhibit angiogenesis by inducing endothelial cell death and by negative regulation of VEGF-induced angiogenesis via caspase-8-mediated enzymatic and non-enzymatic functions. Since NRP1 regulates angiogenesis induced by multiple signals, including VEGF, ECM and semaphorins, and also initiates proliferation of keratinocytes through NF-κB signaling pathway in involved psoriatic skin, targeting NRP1 pathways may offer numerous windows for intervention in psoriasis. In this review, we will focus on the current knowledge about the emerging role of synthetic antimicrobial peptides, TRAIL and NRP1 blocking peptides in the pathogenesis and treatment of psoriasis.
MeSH Terms
-
Anti-Bacterial Agents
Bacteria
Biological Factors
Endothelial Cells
Keratinocytes*
Necrosis
Neuropilin-1
Peptides*
Psoriasis
Receptors, Death Domain
Recurrence
Semaphorins
Skin
Skin Diseases
Staphylococcus
Staphylococcus aureus
Therapeutic Uses*
TNF-Related Apoptosis-Inducing Ligand
United States
Vascular Endothelial Growth Factor A
Anti-Bacterial Agents
Biological Factors
Neuropilin-1
Peptides
Receptors, Death Domain
Semaphorins
TNF-Related Apoptosis-Inducing Ligand
Therapeutic Uses
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. Shbeeb M, Uramoto KM, Gibson LE, O'Fallon WM, Gabriel SE. The epidemiology of psoriatic arthritis in Olmsted County, Minnesota, USA, 1982–1991. J Rheumatol. 2000; 27:1247–1250.2. Boehncke WH, Schön MP. Psoriasis. Lancet. 2015; 386:983–994.
Article3. Javitz HS, Ward MM, Farber E, Nail L, Vallow SG. The direct cost of care for psoriasis and psoriatic arthritis in the United States. J Am Acad Dermatol. 2002; 46:850–860.
Article4. Kurd SK, Gelfand JM. The prevalence of previously diagnosed and undiagnosed psoriasis in US adults: results from NHANES 2003–2004. J Am Acad Dermatol. 2009; 60:218–224.
Article5. Arakawa A, Siewert K, Stöhr J, Besgen P, Kim SM, Rühl G, et al. Melanocyte antigen triggers autoimmunity in human psoriasis. J Exp Med. 2015; 212:2203–2212.
Article6. Lande R, Botti E, Jandus C, Dojcinovic D, Fanelli G, Conrad C, et al. Corrigendum: the antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat Commun. 2015; 6:6595.
Article7. Ono S, Honda T, Doi H, Kabashima K. Concurrence of psoriasis vulgaris and atopic eczema in a single patient exhibiting different expression patterns of psoriatic autoantigens in the lesional skin. JAAD Case Rep. 2018; 4:429–433.
Article8. Wagner EF, Schonthaler HB, Guinea-Viniegra J, Tschachler E. Psoriasis: what we have learned from mouse models. Nat Rev Rheumatol. 2010; 6:704–714.
Article9. Conrad C, Nestle FO. Animal models of psoriasis and psoriatic arthritis: an update. Curr Rheumatol Rep. 2006; 8:342–347.
Article10. Gudjonsson JE, Johnston A, Dyson M, Valdimarsson H, Elder JT. Mouse models of psoriasis. J Invest Dermatol. 2007; 127:1292–1308.
Article11. Menter A. The status of biologic therapies in the treatment of moderate to severe psoriasis. Cutis. 2009; 84:4 Suppl. 14–24.12. Menter A, Gottlieb A, Feldman SR, Van Voorhees AS, Leonardi CL, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008; 58:826–850.
Article13. Lebwohl M, Tyring SK, Hamilton TK, Toth D, Glazer S, Tawfik NH, et al. A novel targeted T-cell modulator, efalizumab, for plaque psoriasis. N Engl J Med. 2003; 349:2004–2013.
Article14. Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007; 445:866–873.
Article15. Griffiths CE, Strober BE, van de Kerkhof P, Ho V, Fidelus-Gort R, Yeilding N, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010; 362:118–128.
Article16. Leonardi CL, Powers JL, Matheson RT, Goffe BS, Zitnik R, Wang A, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003; 349:2014–2022.
Article17. Paller AS, Siegfried EC, Langley RG, Gottlieb AB, Pariser D, Landells I, et al. Etanercept treatment for children and adolescents with plaque psoriasis. N Engl J Med. 2008; 358:241–251.
Article18. Ariza ME, Williams MV, Wong HK. Targeting IL-17 in psoriasis: from cutaneous immunobiology to clinical application. Clin Immunol. 2013; 146:131–139.
Article19. Krueger GG, Langley RG, Leonardi C, Yeilding N, Guzzo C, Wang Y, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007; 356:580–592.
Article20. Leonardi C, Matheson R, Zachariae C, Cameron G, Li L, Edson-Heredia E, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012; 366:1190–1199.
Article21. Lowes MA, Russell CB, Martin DA, Towne JE, Krueger JG. The IL-23/T17 pathogenic axis in psoriasis is amplified by keratinocyte responses. Trends Immunol. 2013; 34:174–181.
Article22. Papp KA, Leonardi C, Menter A, Ortonne JP, Krueger JG, Kricorian G, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012; 366:1181–1189.
Article23. Rich P, Sigurgeirsson B, Thaci D, Ortonne JP, Paul C, Schopf RE, et al. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br J Dermatol. 2013; 168:402–411.
Article24. Waisman A. To be 17 again--anti-interleukin-17 treatment for psoriasis. N Engl J Med. 2012; 366:1251–1252.
Article25. Williams SC. New biologic drugs get under the skin of psoriasis. Nat Med. 2012; 18:638.
Article26. Papp K, Cather JC, Rosoph L, Sofen H, Langley RG, Matheson RT, et al. Efficacy of apremilast in the treatment of moderate to severe psoriasis: a randomised controlled trial. Lancet. 2012; 380:738–746.
Article27. Papp KA, Menter A, Strober B, Langley RG, Buonanno M, Wolk R, et al. Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: a Phase 2b randomized placebo-controlled dose-ranging study. Br J Dermatol. 2012; 167:668–677.
Article28. Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009; 10:207–217.
Article29. Albanesi C, De Pità O, Girolomoni G. Resident skin cells in psoriasis: a special look at the pathogenetic functions of keratinocytes. Clin Dermatol. 2007; 25:581–588.
Article30. Albanesi C, Pastore S. Pathobiology of chronic inflammatory skin diseases: interplay between keratinocytes and immune cells as a target for anti-inflammatory drugs. Curr Drug Metab. 2010; 11:210–227.
Article31. Büchau AS, Gallo RL. Innate immunity and antimicrobial defense systems in psoriasis. Clin Dermatol. 2007; 25:616–624.
Article32. Johnson-Huang LM, McNutt NS, Krueger JG, Lowes MA. Cytokine-producing dendritic cells in the pathogenesis of inflammatory skin diseases. J Clin Immunol. 2009; 29:247–256.
Article33. Zaba LC, Krueger JG, Lowes MA. Resident and “inflammatory” dendritic cells in human skin. J Invest Dermatol. 2009; 129:302–308.
Article34. Ghoreschi K, Weigert C, Röcken M. Immunopathogenesis and role of T cells in psoriasis. Clin Dermatol. 2007; 25:574–580.
Article35. Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009; 129:1339–1350.
Article36. Conrad C, Boyman O, Tonel G, Tun-Kyi A, Laggner U, de Fougerolles A, et al. Alpha1beta1 integrin is crucial for accumulation of epidermal T cells and the development of psoriasis. Nat Med. 2007; 13:836–842.
Article37. Plant D, Young HS, Watson RE, Worthington J, Griffiths CE. The CX3CL1-CX3CR1 system and psoriasis. Exp Dermatol. 2006; 15:900–903.
Article38. Sabat R, Philipp S, Höflich C, Kreutzer S, Wallace E, Asadullah K, et al. Immunopathogenesis of psoriasis. Exp Dermatol. 2007; 16:779–798.
Article39. Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol. 2009; 9:679–691.
Article40. Perera GK, Di Meglio P, Nestle FO. Psoriasis. Annu Rev Pathol. 2012; 7:385–422.
Article41. Albanesi C, Madonna S, Gisondi P, Girolomoni G. The interplay between keratinocytes and immune cells in the pathogenesis of psoriasis. Front Immunol. 2018; 9:1549.
Article42. Morizane S, Gallo RL. Antimicrobial peptides in the pathogenesis of psoriasis. J Dermatol. 2012; 39:225–230.
Article43. Leung DY, Hauk P, Strickland I, Travers JB, Norris DA. The role of superantigens in human diseases: therapeutic implications for the treatment of skin diseases. Br J Dermatol. 1998; 139:Suppl 53. 17–29.
Article44. Henderson CA, Highet AS. Acute psoriasis associated with Lancefield Group C and Group G cutaneous streptococcal infections. Br J Dermatol. 1988; 118:559–561.
Article45. Leung DY, Walsh P, Giorno R, Norris DA. A potential role for superantigens in the pathogenesis of psoriasis. J Invest Dermatol. 1993; 100:225–228.
Article46. Ruíz-González V, Cancino-Diaz JC, Rodríguez-Martínez S, Cancino-Diaz ME. Keratinocytes treated with peptidoglycan from Staphylococcus aureus produce vascular endothelial growth factor, and its expression is amplified by the subsequent production of interleukin-13. Int J Dermatol. 2009; 48:846–854.
Article47. Ezepchuk YV, Leung DY, Middleton MH, Bina P, Reiser R, Norris DA. Staphylococcal toxins and protein A differentially induce cytotoxicity and release of tumor necrosis factor-alpha from human keratinocytes. J Invest Dermatol. 1996; 107:603–609.
Article48. Boehncke WH, Dressel D, Zollner TM, Kaufmann R. Pulling the trigger on psoriasis. Nature. 1996; 379:777.
Article49. Wrone-Smith T, Nickoloff BJ. Dermal injection of immunocytes induces psoriasis. J Clin Invest. 1996; 98:1878–1887.
Article50. Tossi A. Host defense peptides: roles and applications. Curr Protein Pept Sci. 2005; 6:1–3.51. Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002; 415:389–395.
Article52. Koczulla AR, Bals R. Antimicrobial peptides: current status and therapeutic potential. Drugs. 2003; 63:389–406.53. McPhee JB, Hancock RE. Function and therapeutic potential of host defence peptides. J Pept Sci. 2005; 11:677–687.
Article54. Boman HG, Hultmark D. Cell-free immunity in insects. Annu Rev Microbiol. 1987; 41:103–126.
Article55. Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci U S A. 1987; 84:5449–5453.
Article56. Lehrer RI, Ganz T, Selsted ME. Defensins: endogenous antibiotic peptides of animal cells. Cell. 1991; 64:229–230.
Article57. Lai Y, Gallo RL. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009; 30:131–141.
Article58. Ma HL, Liang S, Li J, Napierata L, Brown T, Benoit S, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest. 2008; 118:597–607.
Article59. Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007; 8:950–957.
Article60. Dombrowski Y, Peric M, Koglin S, Kammerbauer C, Göss C, Anz D, et al. Cytosolic DNA triggers inflammasome activation in keratinocytes in psoriatic lesions. Sci Transl Med. 2011; 3:82ra38.
Article61. Secchiero P, Zauli G. Tumor-necrosis-factor-related apoptosis-inducing ligand and the regulation of hematopoiesis. Curr Opin Hematol. 2008; 15:42–48.
Article62. Caldarola G, Carbone A, Arena V, Pennacchia I, De Waure C, Vianale G, et al. Tumour necrosis factor-related apoptosis-inducing ligand (TRAIL): a possible pathogenic role in chronic plaque psoriasis. G Ital Dermatol Venereol. 2016; 151:17–24.63. Johnson-Huang LM, Suárez-Fariñas M, Pierson KC, Fuentes-Duculan J, Cueto I, Lentini T, et al. A single intradermal injection of IFN-γ induces an inflammatory state in both non-lesional psoriatic and healthy skin. J Invest Dermatol. 2012; 132:1177–1187.
Article64. Peternel S, Prpić-Massari L, Manestar-Blažić T, Brajac I, Kaštelan M. Increased expression of TRAIL and its death receptors DR4 and DR5 in plaque psoriasis. Arch Dermatol Res. 2011; 303:389–397.
Article65. Zaba LC, Fuentes-Duculan J, Eungdamrong NJ, Johnson-Huang LM, Nograles KE, White TR, et al. Identification of TNF-related apoptosis-inducing ligand and other molecules that distinguish inflammatory from resident dendritic cells in patients with psoriasis. J Allergy Clin Immunol. 2010; 125:1261–1268.e9.
Article66. Na HJ, Hwang JY, Lee KS, Choi YK, Choe J, Kim JY, et al. TRAIL negatively regulates VEGF-induced angiogenesis via caspase-8-mediated enzymatic and non-enzymatic functions. Angiogenesis. 2014; 17:179–194.
Article67. Saito N, Honma M, Shibuya T, Iinuma S, Igawa S, Kishibe M, et al. RIPK1 downregulation in keratinocyte enhances TRAIL signaling in psoriasis. J Dermatol Sci. 2018; 91:79–86.
Article68. Dannappel M, Vlantis K, Kumari S, Polykratis A, Kim C, Wachsmuth L, et al. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature. 2014; 513:90–94.
Article69. van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009; 182:5836–5845.
Article70. Kawasaki T, Kitsukawa T, Bekku Y, Matsuda Y, Sanbo M, Yagi T, et al. A requirement for neuropilin-1 in embryonic vessel formation. Development. 1999; 126:4895–4902.
Article71. Lampropoulou A, Ruhrberg C. Neuropilin regulation of angiogenesis. Biochem Soc Trans. 2014; 42:1623–1628.
Article72. Schwarz Q, Ruhrberg C. Neuropilin, you gotta let me know: should I stay or should I go? Cell Adh Migr. 2010; 4:61–66.73. Gagnon ML, Bielenberg DR, Gechtman Z, Miao HQ, Takashima S, Soker S, et al. Identification of a natural soluble neuropilin-1 that binds vascular endothelial growth factor: in vivo expression and antitumor activity. Proc Natl Acad Sci U S A. 2000; 97:2573–2578.
Article74. Gu C, Rodriguez ER, Reimert DV, Shu T, Fritzsch B, Richards LJ, et al. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell. 2003; 5:45–57.
Article75. Nakamura F, Kalb RG, Strittmatter SM. Molecular basis of semaphorin-mediated axon guidance. J Neurobiol. 2000; 44:219–229.
Article76. Rossignol M, Gagnon ML, Klagsbrun M. Genomic organization of human neuropilin-1 and neuropilin-2 genes: identification and distribution of splice variants and soluble isoforms. Genomics. 2000; 70:211–222.
Article77. He Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell. 1997; 90:739–751.
Article78. Kolodkin AL, Ginty DD. Steering clear of semaphorins: neuropilins sound the retreat. Neuron. 1997; 19:1159–1162.
Article79. Soker S, Fidder H, Neufeld G, Klagsbrun M. Characterization of novel vascular endothelial growth factor (VEGF) receptors on tumor cells that bind VEGF165 via its exon 7-encoded domain. J Biol Chem. 1996; 271:5761–5767.
Article80. Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998; 92:735–745.
Article81. Chen H, Chédotal A, He Z, Goodman CS, Tessier-Lavigne M. Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins Sema E and Sema IV but not Sema III. Neuron. 1997; 19:547–559.
Article82. Giger RJ, Urquhart ER, Gillespie SK, Levengood DV, Ginty DD, Kolodkin AL. Neuropilin-2 is a receptor for semaphorin IV: insight into the structural basis of receptor function and specificity. Neuron. 1998; 21:1079–1092.
Article83. Gluzman-Poltorak Z, Cohen T, Herzog Y, Neufeld G. Neuropilin-2 is a receptor for the vascular endothelial growth factor (VEGF) forms VEGF-145 and VEGF-165 [corrected]. J Biol Chem. 2000; 275:18040–18045.
Article84. Gu C, Limberg BJ, Whitaker GB, Perman B, Leahy DJ, Rosenbaum JS, et al. Characterization of neuropilin-1 structural features that confer binding to semaphorin 3A and vascular endothelial growth factor 165. J Biol Chem. 2002; 277:18069–18076.
Article85. Parker MW, Hellman LM, Xu P, Fried MG, Vander Kooi CW. Furin processing of semaphorin 3F determines its anti-angiogenic activity by regulating direct binding and competition for neuropilin. Biochemistry. 2010; 49:4068–4075.
Article86. Henno A, Blacher S, Lambert C, Colige A, Seidel L, Noël A, et al. Altered expression of angiogenesis and lymphangiogenesis markers in the uninvolved skin of plaque-type psoriasis. Br J Dermatol. 2009; 160:581–590.
Article87. Henno A, Blacher S, Lambert CA, Deroanne C, Noël A, Lapière C, et al. Histological and transcriptional study of angiogenesis and lymphangiogenesis in uninvolved skin, acute pinpoint lesions and established psoriasis plaques: an approach of vascular development chronology in psoriasis. J Dermatol Sci. 2010; 57:162–169.
Article88. Saban MR, Sferra TJ, Davis CA, Simpson C, Allen A, Maier J, et al. Neuropilin-VEGF signaling pathway acts as a key modulator of vascular, lymphatic, and inflammatory cell responses of the bladder to intravesical BCG treatment. Am J Physiol Renal Physiol. 2010; 299:F1245–F1256.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antimicrobial Peptides in Innate Immunity against Mycobacteria
- Identification of Antimicrobial Peptide Hexamers against Oral Pathogens through Rapid Screening of a Synthetic Combinatorial Peptide Library
- Expression of Antimicrobial Peptides and Proteins in Epidermis Equivalents Exposed to Salt Water and Narrowband Ultraviolet B Radiation
- Psoriasis as a T-cell-mediated Immunologic Disease
- Recent Trends in Cyclic Peptides as Therapeutic Agents and Biochemical Tools