Clin Nutr Res.
2019 Jan;8(1):17-27. 10.7762/cnr.2019.8.1.17.
Effect of Eicosapentaenoic Acid Supplementation on Paraoxonase 2 Gene Expression in Patients with Type 2 Diabetes Mellitus: a Randomized Double-blind Clinical Trial
- Affiliations
-
- 1Department of Cellular and Molecular Nutrition, School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences, Tehran 14155-6446, Iran. mjalali87@yahoo.com
- 2Student Research Committee, Department of Clinical Nutrition, School of Nutrition and Food Sciences, Isfahan University of Medical Sciences, Isfahan 81746-73461, Iran.
- KMID: 2444235
- DOI: http://doi.org/10.7762/cnr.2019.8.1.17
Abstract
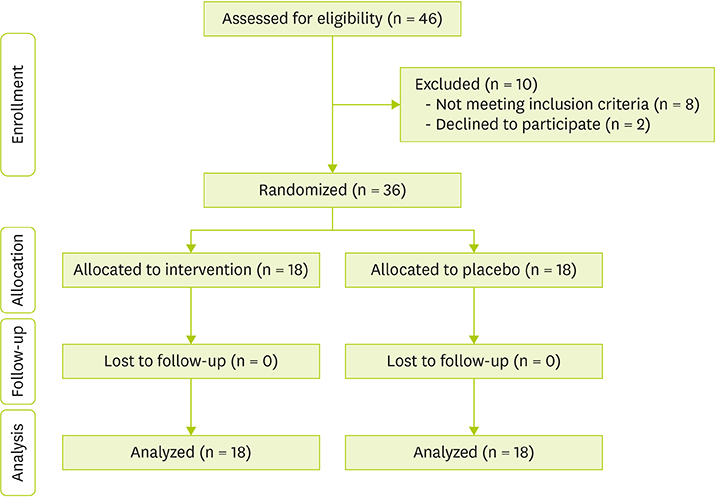
- Type 2 diabetes mellitus (T2DM) is recognized as one of the most prevalent metabolic diseases, and it is mostly associated with oxidative stress, atherosclerosis and dyslipidemia. Paraoxonase 2 (PON2) due to its antioxidant properties may play a role in the atherosclerosis development. Although long-chain omega-3 polyunsaturated fatty acids, such as eicosapentaenoic acid (EPA) have been shown to reduce the risk of cardiovascular disease, the exact mechanism of action is still unknown. Our goal in this study was to determine the effect of EPA administration on gene expression of PON2 in patients with T2DM. Present study was a randomized, controlled double-blind trial. Thirty-six patients with T2DM were randomly allocated to receive 2 g/day EPA (n = 18) or placebo (n = 18) for 8 weeks. There were no significant differences between 2 groups concerning demographic or biochemical variables, and dietary intakes as well (p > 0.05). However, patients received EPA showed a significant increase in the gene expression of PON2 compared with placebo group (p = 0.027). In addition, high-density lipoprotein cholesterol increased and fasting blood sugar decreased significantly after EPA supplementation compared with control group. Taken together, supplementation with 2 g/day EPA could be atheroprotective via the upregulation of PON2 in patients with T2DM. TRIAL REGISTRATION: ClinicalTrials.gov Identifier: NCT03258840
Keyword
MeSH Terms
-
Aryldialkylphosphatase*
Atherosclerosis
Blood Glucose
Cardiovascular Diseases
Cholesterol
Diabetes Mellitus, Type 2*
Dyslipidemias
Eicosapentaenoic Acid*
Fasting
Fatty Acids, Unsaturated
Gene Expression*
Humans
Lipoproteins
Metabolic Diseases
Oxidative Stress
Up-Regulation
Aryldialkylphosphatase
Blood Glucose
Cholesterol
Eicosapentaenoic Acid
Fatty Acids, Unsaturated
Lipoproteins
Figure
Reference
-
1. Blair M. Diabetes mellitus review. Urol Nurs. 2016; 36:27–36.
Article2. Chang YC, Chuang LM. The role of oxidative stress in the pathogenesis of type 2 diabetes: from molecular mechanism to clinical implication. Am J Transl Res. 2010; 2:316–331.3. Rathmann W, Giani G. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004; 27:2568–2569.
Article4. International Diabetes Federation. Diabetes atlas. 3rd ed. Brussels: International Diabetes Federation;2006.5. Chan GC, Tang SC. Diabetic nephropathy: landmark clinical trials and tribulations. Nephrol Dial Transplant. 2016; 31:359–368.
Article6. Ahangarpour A, Heidari H, Junghani MS, Absari R, Khoogar M, Ghaedi E. Effects of hydroalcoholic extract of Rhus coriaria seed on glucose and insulin related biomarkers, lipid profile, and hepatic enzymes in nicotinamide-streptozotocin-induced type II diabetic male mice. Res Pharm Sci. 2017; 12:416–424.7. Bhattacharyya T, Nicholls SJ, Topol EJ, Zhang R, Yang X, Schmitt D, Fu X, Shao M, Brennan DM, Ellis SG, Brennan ML, Allayee H, Lusis AJ, Hazen SL. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA. 2008; 299:1265–1276.
Article8. Draganov DI, La Du BN. Pharmacogenetics of paraoxonases: a brief review. Naunyn Schmiedebergs Arch Pharmacol. 2004; 369:78–88.
Article9. Ng CJ, Shih DM, Hama SY, Villa N, Navab M, Reddy ST. The paraoxonase gene family and atherosclerosis. Free Radic Biol Med. 2005; 38:153–163.
Article10. Rosenblat M, Draganov D, Watson CE, Bisgaier CL, La Du BN, Aviram M. Mouse macrophage paraoxonase 2 activity is increased whereas cellular paraoxonase 3 activity is decreased under oxidative stress. Arterioscler Thromb Vasc Biol. 2003; 23:468–474.
Article11. Levy E, Trudel K, Bendayan M, Seidman E, Delvin E, Elchebly M, Lavoie JC, Precourt LP, Amre D, Sinnett D. Biological role, protein expression, subcellular localization, and oxidative stress response of paraoxonase 2 in the intestine of humans and rats. Am J Physiol Gastrointest Liver Physiol. 2007; 293:G1252–G1261.
Article12. Draganov DI, Teiber JF, Speelman A, Osawa Y, Sunahara R, La Du BN. Human paraoxonases (PON1, PON2, and PON3) are lactonases with overlapping and distinct substrate specificities. J Lipid Res. 2005; 46:1239–1247.
Article13. Précourt LP, Amre D, Denis MC, Lavoie JC, Delvin E, Seidman E, Levy E. The three-gene paraoxonase family: physiologic roles, actions and regulation. Atherosclerosis. 2011; 214:20–36.
Article14. Kesavulu MM, Kameswararao B, Apparao C, Kumar EG, Harinarayan CV. Effect of omega-3 fatty acids on lipid peroxidation and antioxidant enzyme status in type 2 diabetic patients. Diabetes Metab. 2002; 28:20–26.15. Figueras M, Olivan M, Busquets S, López-Soriano FJ, Argilés JM. Effects of eicosapentaenoic acid (EPA) treatment on insulin sensitivity in an animal model of diabetes: improvement of the inflammatory status. Obesity (Silver Spring). 2011; 19:362–369.
Article16. Terano T, Hirai A, Hamazaki T, Kobayashi S, Fujita T, Tamura Y, Kumagai A. Effect of oral administration of highly purified eicosapentaenoic acid on platelet function, blood viscosity and red cell deformability in healthy human subjects. Atherosclerosis. 1983; 46:321–331.
Article17. Dyerberg J, Bang HO, Stoffersen E, Moncada S, Vane JR. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet. 1978; 2:117–119.
Article18. Hagiwara S, Makita Y, Gu L, Tanimoto M, Zhang M, Nakamura S, Kaneko S, Itoh T, Gohda T, Horikoshi S, Tomino Y. Eicosapentaenoic acid ameliorates diabetic nephropathy of type 2 diabetic KKAy/Ta mice: involvement of MCP-1 suppression and decreased ERK1/2 and p38 phosphorylation. Nephrol Dial Transplant. 2006; 21:605–615.
Article19. Dias CB, Amigo N, Wood LG, Correig X, Garg ML. Effect of diets rich in either saturated fat or n-6 polyunsaturated fatty acids and supplemented with long-chain n-3 polyunsaturated fatty acids on plasma lipoprotein profiles. Eur J Clin Nutr. 2017; 71:1297–1302.
Article20. Tajima-Shirasaki N, Ishii KA, Takayama H, Shirasaki T, Iwama H, Chikamoto K, Saito Y, Iwasaki Y, Teraguchi A, Lan F, Kikuchi A, Takeshita Y, Murao K, Matsugo S, Kaneko S, Misu H, Takamura T. Eicosapentaenoic acid down-regulates expression of the selenoprotein P gene by inhibiting SREBP-1c protein independently of the AMP-activated protein kinase pathway in H4IIEC3 hepatocytes. J Biol Chem. 2017; 292:10791–10800.
Article21. Mansoori A, Sotoudeh G, Djalali M, Eshraghian MR, Keramatipour M, Nasli-Esfahani E, Shidfar F, Alvandi E, Toupchian O, Koohdani F. Effect of DHA-rich fish oil on PPARγ target genes related to lipid metabolism in type 2 diabetes: a randomized, double-blind, placebo-controlled clinical trial. J Clin Lipidol. 2015; 9:770–777.
Article22. Peet M, Horrobin DF. E-E Multicentre Study Group. A dose-ranging exploratory study of the effects of ethyl-eicosapentaenoate in patients with persistent schizophrenic symptoms. J Psychiatr Res. 2002; 36:7–18.
Article23. Summary of revisions for the 2010 Clinical Practice Recommendations. Diabetes Care. 2010; 33:Suppl 1. S3.24. Alberti KG, Zimmet P, Shaw J. International Diabetes Federation: a consensus on type 2 diabetes prevention. Diabet Med. 2007; 24:451–463.
Article25. Kwok S, Higuchi R. Avoiding false positives with PCR. Nature. 1989; 339:237–238.
Article26. Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. In : Misener S, Krawetz SA, editors. Bioinformatics methods and protocols. New York (NY): Springer/Humana Press;1999. p. 365–386.27. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001; 29:e45.
Article28. Ghorbanihaghjo A, Kolahi S, Seifirad S, Rashtchizadeh N, Argani H, Hajialilo M, Khabazi A, Alizadeh S, Bahreini E. Effect of fish oil supplements on serum paraoxonase activity in female patients with rheumatoid arthritis: a double-blind randomized controlled trial. Arch Iran Med. 2012; 15:549–552.29. Miljkovic M, Djuricic I, Kotur-Stevuljevic J, Sobajic S, Spasojevic-Kalimanovska V, Jelic-Ivanovic Z, Kerkez M, Djordjevic V, Djurasic L, Spasic S. Omega-3 fatty acids supplementation effects on paraoxonase-1 enzymatic activity. J Food Nutr Res. 2015; 54:314–322.30. Singer P, Jaeger W, Wirth M, Voigt S, Naumann E, Zimontkowski S, Hajdu I, Goedicke W. Lipid and blood pressure-lowering effect of mackerel diet in man. Atherosclerosis. 1983; 49:99–108.
Article31. Woodman RJ, Mori TA, Burke V, Puddey IB, Watts GF, Beilin LJ. Effects of purified eicosapentaenoic and docosahexaenoic acids on glycemic control, blood pressure, and serum lipids in type 2 diabetic patients with treated hypertension. Am J Clin Nutr. 2002; 76:1007–1015.
Article32. Luo J, Rizkalla SW, Vidal H, Oppert JM, Colas C, Boussairi A, Guerre-Millo M, Chapuis AS, Chevalier A, Durand G, Slama G. Moderate intake of n-3 fatty acids for 2 months has no detrimental effect on glucose metabolism and could ameliorate the lipid profile in type 2 diabetic men. Results of a controlled study. Diabetes Care. 1998; 21:717–724.
Article33. Okuda Y, Kawashima K, Sawada T, Tsurumaru K, Asano M, Suzuki S, Soma M, Nakajima T, Yamashita K. Eicosapentaenoic acid enhances nitric oxide production by cultured human endothelial cells. Biochem Biophys Res Commun. 1997; 232:487–491.
Article34. Okumura T, Fujioka Y, Morimoto S, Tsuboi S, Masai M, Tsujino T, Ohyanagi M, Iwasaki T. Eicosapentaenoic acid improves endothelial function in hypertriglyceridemic subjects despite increased lipid oxidizability. Am J Med Sci. 2002; 324:247–253.
Article35. Tamura Y, Hirai A, Terano T, Takenaga M, Saitoh H, Tahara K, Yoshida S. Clinical and epidemiological studies of eicosapentaenoic acid (EPA) in Japan. Prog Lipid Res. 1986; 25:461–466.
Article36. Thies F, Garry JM, Yaqoob P, Rerkasem K, Williams J, Shearman CP, Gallagher PJ, Calder PC, Grimble RF. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomised controlled trial. Lancet. 2003; 361:477–485.
Article37. Motti C, Dessì M, Gnasso A, Irace C, Indigeno P, Angelucci CB, Bernardini S, Fucci G, Federici G, Cortese C. A multiplex PCR-based DNA assay for the detection of paraoxonase gene cluster polymorphisms. Atherosclerosis. 2001; 158:35–40.
Article38. Ng CJ, Wadleigh DJ, Gangopadhyay A, Hama S, Grijalva VR, Navab M, Fogelman AM, Reddy ST. Paraoxonase-2 is a ubiquitously expressed protein with antioxidant properties and is capable of preventing cell-mediated oxidative modification of low density lipoprotein. J Biol Chem. 2001; 276:44444–44449.
Article39. Rosenblat M, Coleman R, Reddy ST, Aviram M. Paraoxonase 2 attenuates macrophage triglyceride accumulation via inhibition of diacylglycerol acyltransferase 1. J Lipid Res. 2009; 50:870–879.
Article40. Fuhrman B, Volkova N, Aviram M. Oxidative stress increases the expression of the CD36 scavenger receptor and the cellular uptake of oxidized low-density lipoprotein in macrophages from atherosclerotic mice: protective role of antioxidants and of paraoxonase. Atherosclerosis. 2002; 161:307–316.
Article41. Shiner M, Fuhrman B, Aviram M. Paraoxonase 2 (PON2) expression is upregulated via a reduced-nicotinamide-adenine-dinucleotide-phosphate (NADPH)-oxidase-dependent mechanism during monocytes differentiation into macrophages. Free Radic Biol Med. 2004; 37:2052–2063.
Article42. Altenhöfer S, Witte I, Teiber JF, Wilgenbus P, Pautz A, Li H, Daiber A, Witan H, Clement AM, Förstermann U, Horke S. One enzyme, two functions: PON2 prevents mitochondrial superoxide formation and apoptosis independent from its lactonase activity. J Biol Chem. 2010; 285:24398–24403.43. Ng CJ, Hama SY, Bourquard N, Navab M, Reddy ST. Adenovirus mediated expression of human paraoxonase 2 protects against the development of atherosclerosis in apolipoprotein E-deficient mice. Mol Genet Metab. 2006; 89:368–373.
Article44. Mackness B, McElduff P, Mackness MI. The paraoxonase-2-310 polymorphism is associated with the presence of microvascular complications in diabetes mellitus. J Intern Med. 2005; 258:363–368.
Article45. Leus FR, Zwart M, Kastelein JJ, Voorbij HA. PON2 gene variants are associated with clinical manifestations of cardiovascular disease in familial hypercholesterolemia patients. Atherosclerosis. 2001; 154:641–649.
Article46. Kao Y, Donaghue KC, Chan A, Bennetts BH, Knight J, Silink M. Paraoxonase gene cluster is a genetic marker for early microvascular complications in type 1 diabetes. Diabet Med. 2002; 19:212–215.
Article47. Rasic-Milutinovic Z, Popovic T, Perunicic-Pekovic G, Arsic A, Borozan S, Glibetic M. Lower serum paraoxonase-1 activity is related to linoleic and docosahexanoic fatty acids in type 2 diabetic patients. Arch Med Res. 2012; 43:75–82.
Article48. Haraguchi Y, Toh R, Hasokawa M, Nakajima H, Honjo T, Otsui K, Mori K, Miyamoto-Sasaki M, Shinohara M, Nishimura K, Ishida T, Hirata K. Serum myeloperoxidase/paraoxonase 1 ratio as potential indicator of dysfunctional high-density lipoprotein and risk stratification in coronary artery disease. Atherosclerosis. 2014; 234:288–294.
Article49. Zarei M, Fakher S, Tabei SM, Javanbakht MH, Derakhshanian H, Farahbakhsh-Farsi P, Sadeghi MR, Mostafavi E, Djalali M. Effects of vitamin A, C and E, or omega-3 fatty acid supplementation on the level of paraoxonase and arylesterase activity in streptozotocin-induced diabetic rats: an investigation of activities in plasma, and heart and liver homogenates. Singapore Med J. 2016; 57:153–156.
Article50. Calabresi L, Villa B, Canavesi M, Sirtori CR, James RW, Bernini F, Franceschini G. An ω-3 polyunsaturated fatty acid concentrate increases plasma high-density lipoprotein 2 cholesterol and paraoxonase levels in patients with familial combined hyperlipidemia. Metabolism. 2004; 53:153–158.
Article51. Baskol G, Demir H, Baskol M, Kilic E, Ates F, Kocer D, Muhtaroglu S. Assessment of paraoxonase 1 activity and malondialdehyde levels in patients with rheumatoid arthritis. Clin Biochem. 2005; 38:951–955.
Article52. Burillo E, Mateo-Gallego R, Cenarro A, Fiddyment S, Bea AM, Jorge I, Vázquez J, Civeira F. Beneficial effects of omega-3 fatty acids in the proteome of high-density lipoprotein proteome. Lipids Health Dis. 2012; 11:116.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Omega-3 Supplementation on Adipocytokines in Prediabetes and Type 2 Diabetes Mellitus: Systematic Review and Meta-Analysis of Randomized Controlled Trials
- Plasma Paraoxonase Activities in Type 2 Diabetes Mellitus
- Effect of Selenium Supplementation on Expression of SIRT1 and PGC-1α Genes in Ulcerative Colitis Patients: a Double Blind Randomized Clinical Trial
- Effects of folic acid supplementation on serum homocysteine levels, lipid profiles, and vascular parameters in post-menopausal Korean women with type 2 diabetes mellitus
- Letter: Efficacy and Safety of Voglibose Plus Metformin in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial (Diabetes Metab J 2019;43;276-86)