Korean J Physiol Pharmacol.
2019 May;23(3):171-179. 10.4196/kjpp.2019.23.3.171.
MicroRNA-186 targets SKP2 to induce p27(Kip1)-mediated pituitary tumor cell cycle deregulation and modulate cell proliferation
- Affiliations
-
- 1Department of Neurosurgery, Sichuan Academy of Medical Sciences and Sichuan Provincial People's Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu 610072, Sichuan, China. chenly11@163.com, tangjian@med.uestc.edu.cn
- KMID: 2443605
- DOI: http://doi.org/10.4196/kjpp.2019.23.3.171
Abstract
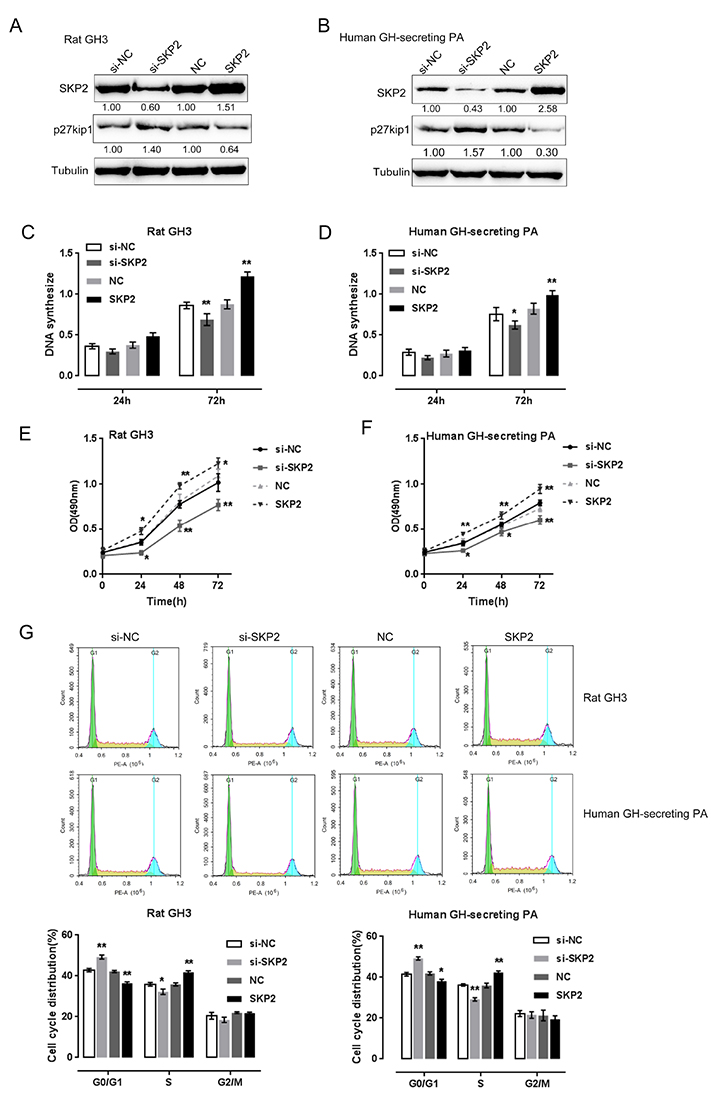
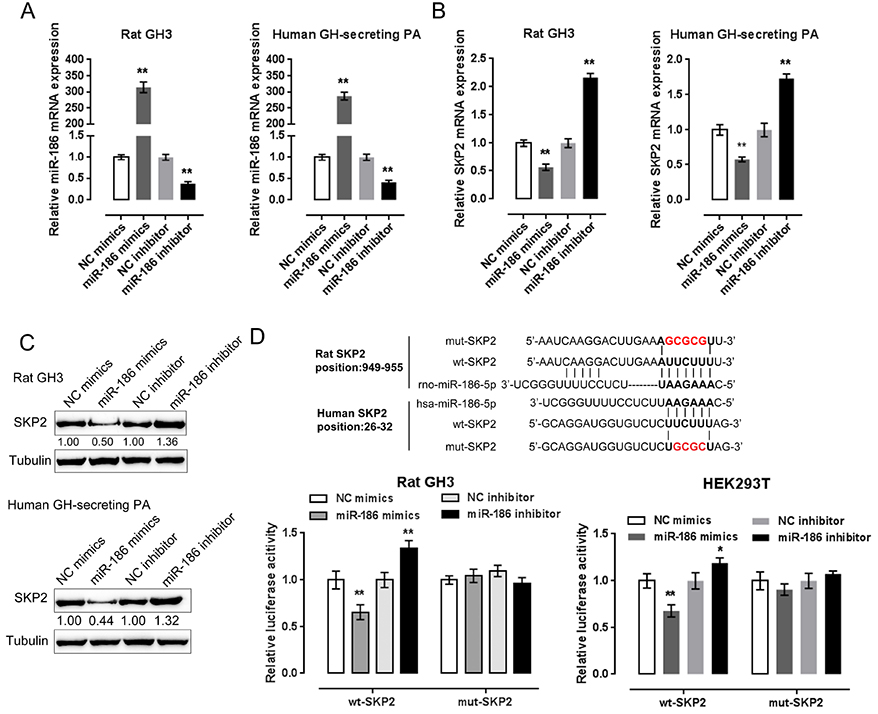
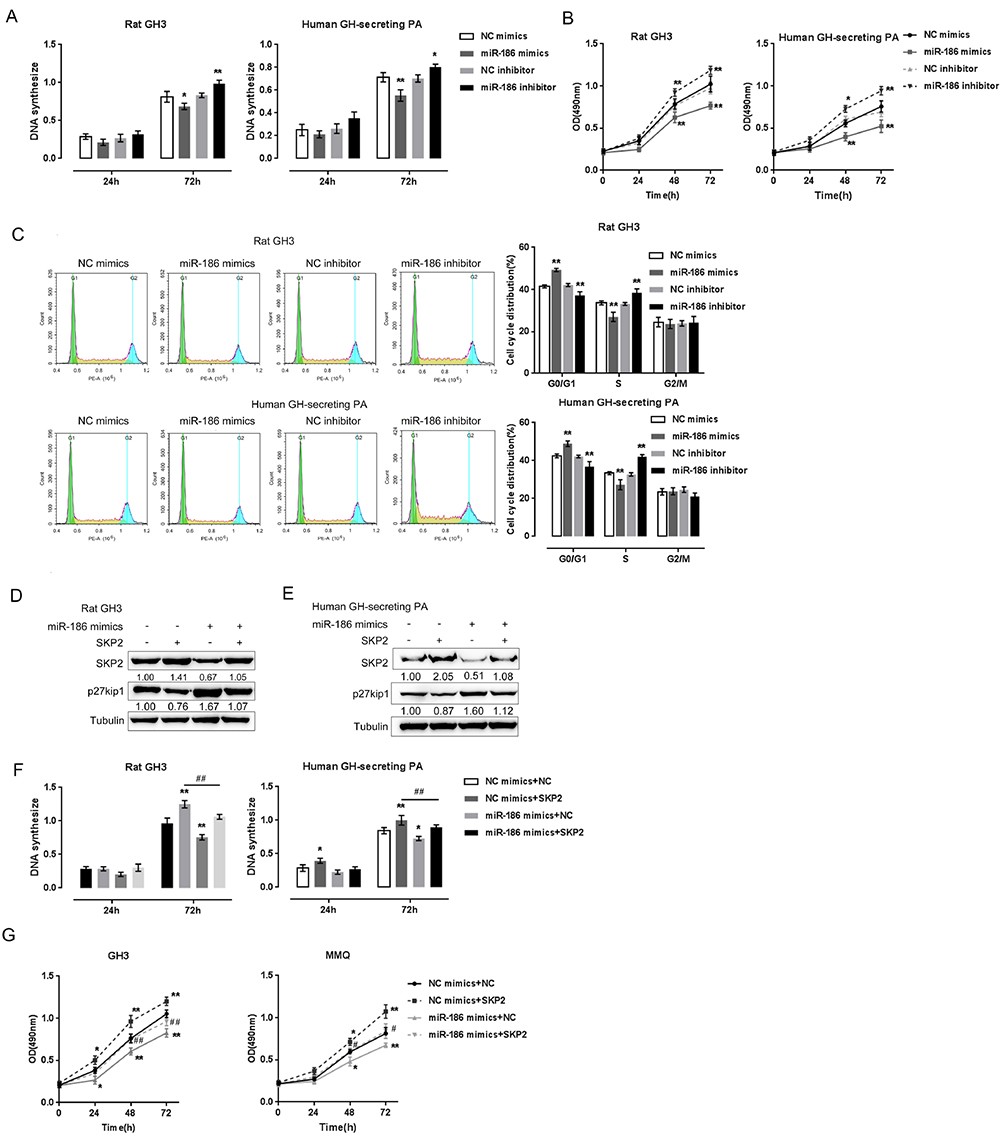
- Pituitary tumors are usually benign but can occasionally exhibit hormonal and proliferative behaviors. Dysregulation of the G1/S restriction point largely contributes to the over-proliferation of pituitary tumor cells. F-box protein S-phase kinase-interacting protein-2 (SKP2) reportedly targets and inhibits the expression of p27(Kip1), a well-known negative regulator of G1 cell cycle progression. In this study, SKP2 expression was found to be upregulated while p27(Kip1) expression was determined to be downregulated in rat and human pituitary tumor cells. Furthermore, SKP2 knockdown induced upregulation of p27(Kip1) and cell growth inhibition in rat and human pituitary tumor cells, while SKP2overexpression elicited opposite effects on p27(Kip1) expression and cell growth. The expression of microRNA-186 (miR-186) was reported to be reduced in pituitary tumors. Online tools predicted SKP2 to be a direct downstream target of miR-186, which was further confirmed by luciferase reporter gene assays. Moreover, miR-186 could modulate the cell proliferation and p27(Kip1)-mediated cell cycle alternation of rat and human pituitary tumor cells through SKP2. As further confirmation of these findings, miR-186 and p27(Kip1) expression were downregulated, while SKP2 expression was upregulated in human pituitary tumor tissue samples; thus, SKP2 expression negatively correlated with miR-186 and p27(Kip1) expression. In contrast, miR-186 expression positively associated with p27(Kip1) expression. Taken together, we discovered a novel mechanism by which miR-186/SKP2 axis modulates pituitary tumor cell proliferation through p27(Kip1)-mediated cell cycle alternation.
Keyword
MeSH Terms
Figure
Reference
-
1. Shimon I, Melmed S. Genetic basis of endocrine disease: pituitary tumor pathogenesis. J Clin Endocrinol Metab. 1997; 82:1675–1681.2. Coire CI, Horvath E, Kovacs K, Smyth HS, Ezzat S. Cushing's syndrome from an ectopic pituitary adenoma with peliosis: a histological, immunohistochemical, and ultrastructural study and review of the literature. Endocr Pathol. 1997; 8:65–74.
Article3. Asa SL, Ezzat S. The cytogenesis and pathogenesis of pituitary adenomas. Endocr Rev. 1998; 19:798–827.
Article4. Palumbo T, Faucz FR, Azevedo M, Xekouki P, Iliopoulos D, Stratakis CA. Functional screen analysis reveals miR-26b and miR-128 as central regulators of pituitary somatomammotrophic tumor growth through activation of the PTEN-AKT pathway. Oncogene. 2013; 32:1651–1659.
Article5. Tang FY, Shih CJ, Cheng LH, Ho HJ, Chen HJ. Lycopene inhibits growth of human colon cancer cells via suppression of the Akt signaling pathway. Mol Nutr Food Res. 2008; 52:646–654.6. Li W, Zhang G, Wang HL, Wang L. Analysis of expression of cyclin E, p27kip1 and Ki67 protein in colorectal cancer tissues and its value for diagnosis, treatment and prognosis of disease. Eur Rev Med Pharmacol Sci. 2016; 20:4874–4879.7. Berton S, Cusan M, Segatto I, Citron F, D'Andrea S, Benevol S, Avanzo M, Dall'Acqua A, Schiappacassi M, Bristow RG, Belletti B, Baldassarre G. Loss of p27kip1 increases genomic instability and induces radio-resistance in luminal breast cancer cells. Sci Rep. 2017; 7:595.
Article8. Kim GJ, Kim DH, Min KW, Kim YH, Oh YH. Loss of p27kip1 expression is associated with poor prognosis in patients with taxane-treated breast cancer. Pathol Res Pract. 2018; 214:565–571.9. Haddad NF, Teodoro AJ, Leite de Oliveira F, Soares N, de Mattos RM, Hecht F, Dezonne RS, Vairo L, Goldenberg RC, Gomes FC, de Carvalho DP, Gadelha MR, Nasciutti LE, Miranda-Alves L. Lycopene and beta-carotene induce growth inhibition and proapoptotic effects on ACTH-secreting pituitary adenoma cells. PLoS One. 2013; 8:e62773.
Article10. Kulinski M, Achkar IW, Haris M, Dermime S, Mohammad RM, Uddin S. Dysregulated expression of SKP2 and its role in hematological malignancies. Leuk Lymphoma. 2018; 59:1051–1063.
Article11. Ma Y, Yan M, Huang H, Zhang L, Wang Q, Zhao Y, Zhao J. Associations and prognostic significance of p27Kip1, Jab1 and Skp2 in non-Hodgkin lymphoma. Mol Clin Oncol. 2016; 5:357–364.12. Borriello A, Naviglio S, Bencivenga D, Caldarelli I, Tramontano A, Speranza MC, Stampone E, Sapio L, Negri A, Oliva A, Sinisi AA, Spina A, Della Ragione F. Histone deacetylase inhibitors increase p27(Kip1) by affecting its ubiquitin-dependent degradation through Skp2 downregulation. Oxid Med Cell Longev. 2016; 2016:2481865.13. Frescas D, Pagano M. Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the scales of cancer. Nat Rev Cancer. 2008; 8:438–449.14. Croce CM, Calin GA. miRNAs, cancer, and stem cell division. Cell. 2005; 122:6–7.
Article15. Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M, Ménard S, Palazzo JP, Rosenberg A, Musiani P, Volinia S, Nenci I, Calin GA, Querzoli P, Negrini M, Croce CM. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005; 65:7065–7070.
Article16. Bandres E, Bitarte N, Arias F, Agorreta J, Fortes P, Agirre X, Zarate R, Diaz-Gonzalez JA, Ramirez N, Sola JJ, Jimenez P, Rodriguez J, Garcia-Foncillas J. microRNA-451 regulates macrophage migration inhibitory factor production and proliferation of gastrointestinal cancer cells. Clin Cancer Res. 2009; 15:2281–2290.
Article17. Stilling G, Sun Z, Zhang S, Jin L, Righi A, Kovācs G, Korbonits M, Scheithauer BW, Kovacs K, Lloyd RV. MicroRNA expression in ACTH-producing pituitary tumors: up-regulation of microRNA-122 and -493 in pituitary carcinomas. Endocrine. 2010; 38:67–75.
Article18. Bottoni A, Piccin D, Tagliati F, Luchin A, Zatelli MC, degli Uberti EC. miR-15a and miR-16-1 down-regulation in pituitary adenomas. J Cell Physiol. 2005; 204:280–285.
Article19. Wierinckx A, Roche M, Legras-Lachuer C, Trouillas J, Raverot G, Lachuer J. MicroRNAs in pituitary tumors. Mol Cell Endocrinol. 2017; 456:51–61.
Article20. Zhang QJ, Xu C. The role of microRNAs in the pathogenesis of pituitary tumors. Front Biosci (Landmark Ed). 2016; 21:1–7.
Article21. Sapochnik M, Nieto LE, Fuertes M, Arzt E. Molecular mechanisms underlying pituitary pathogenesis. Biochem Genet. 2016; 54:107–119.
Article22. Dweep H, Gretz N, Sticht C. miRWalk database for miRNA-target interactions. Methods Mol Biol. 2014; 1182:289–305.
Article23. John B, Enright AJ, Aravin A, Tuschl T, Sander C, Marks DS. Human MicroRNA targets. PLoS Biol. 2004; 2:e363.
Article24. Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. Elife. 2015; 4:e05005.
Article25. Molatore S, Marinoni I, Lee M, Pulz E, Ambrosio MR, degli Uberti EC, Zatelli MC, Pellegata NS. A novel germline CDKN1B mutation causing multiple endocrine tumors: clinical, genetic and functional characterization. Hum Mutat. 2010; 31:E1825–E1835.
Article26. An J, Pei X, Zang Z, Zhou Z, Hu J, Zheng X, Zhang Y, He J, Duan L, Shen R, Zhang W, Zhu F, Li S, Yang H. Metformin inhibits proliferation and growth hormone secretion of GH3 pituitary adenoma cells. Oncotarget. 2017; 8:37538–37549.
Article27. Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995; 9:1149–1163.
Article28. Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999; 13:1501–1512.
Article29. Muşat M, Vax VV, Borboli N, Gueorguiev M, Bonner S, Korbonits M, Grossman AB. Cell cycle dysregulation in pituitary oncogenesis. Front Horm Res. 2004; 32:34–62.30. Craig C, Wersto R, Kim M, Ohri E, Li Z, Katayose D, Lee SJ, Trepel J, Cowan K, Seth P. A recombinant adenovirus expressing p27Kip1 induces cell cycle arrest and loss of cyclin-Cdk activity in human breast cancer cells. Oncogene. 1997; 14:2283–2289.
Article31. Katayose Y, Kim M, Rakkar AN, Li Z, Cowan KH, Seth P. Promoting apoptosis: a novel activity associated with the cyclin-dependent kinase inhibitor p27. Cancer Res. 1997; 57:5441–5445.32. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004; 116:281–297.33. Liang HQ, Wang RJ, Diao CF, Li JW, Su JL, Zhang S. The PTTG1-targeting miRNAs miR-329, miR-300, miR-381, and miR-655 inhibit pituitary tumor cell tumorigenesis and are involved in a p53/PTTG1 regulation feedback loop. Oncotarget. 2015; 6:29413–29427.
Article34. Renjie W, Haiqian L. MiR-132, miR-15a and miR-16 synergistically inhibit pituitary tumor cell proliferation, invasion and migration by targeting Sox5. Cancer Lett. 2015; 356:568–578.
Article35. Cai J, Wu J, Zhang H, Fang L, Huang Y, Yang Y, Zhu X, Li R, Li M. miR-186 downregulation correlates with poor survival in lung adenocarcinoma, where it interferes with cell-cycle regulation. Cancer Res. 2013; 73:756–766.
Article36. Yang J, Yuan D, Li J, Zheng S, Wang B. miR-186 downregulates protein phosphatase PPM1B in bladder cancer and mediates G1-S phase transition. Tumour Biol. 2016; 37:4331–4341.
Article37. Hua X, Xiao Y, Pan W, Li M, Huang X, Liao Z, Xian Q, Yu L. miR-186 inhibits cell proliferation of prostate cancer by targeting GOLPH3. Am J Cancer Res. 2016; 6:1650–1660.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Relevance of Women's Diseases, Jun Activation-domain Binding Protein 1 (JAB1) and p27(kip1)
- The function of p27(KIP1) during tumor development
- Prognostic Implications of Cyclin B1, p34cdc2, p27(Kip1) and p53 Expression in Gastric Cancer
- p27(Kip1) and Ki-67 Expression in Mucoepidermoid Carcinoma
- Alteration in Expression of p27(Kip1) in Mouse Vestibular Epithelia during Apoptosis Induced by Gentamicin