Nat Prod Sci.
2019 Mar;25(1):23-27. 10.20307/nps.2019.25.1.23.
Cytotoxic Lactones from the Pericarps of Litsea japonica
- Affiliations
-
- 1College of Pharmacy, Drug Research and Development Center, Daegu Catholic University, Gyeongbuk 38430, Republic of Korea. bsmin@cu.ac.kr
- KMID: 2443099
- DOI: http://doi.org/10.20307/nps.2019.25.1.23
Abstract
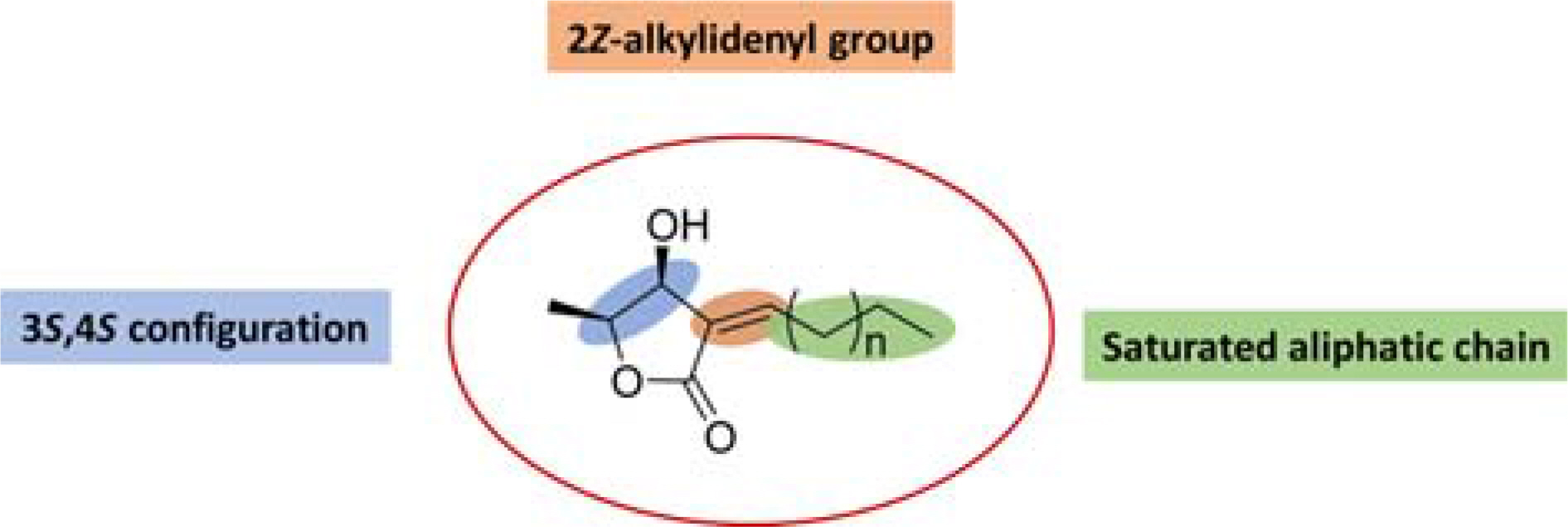
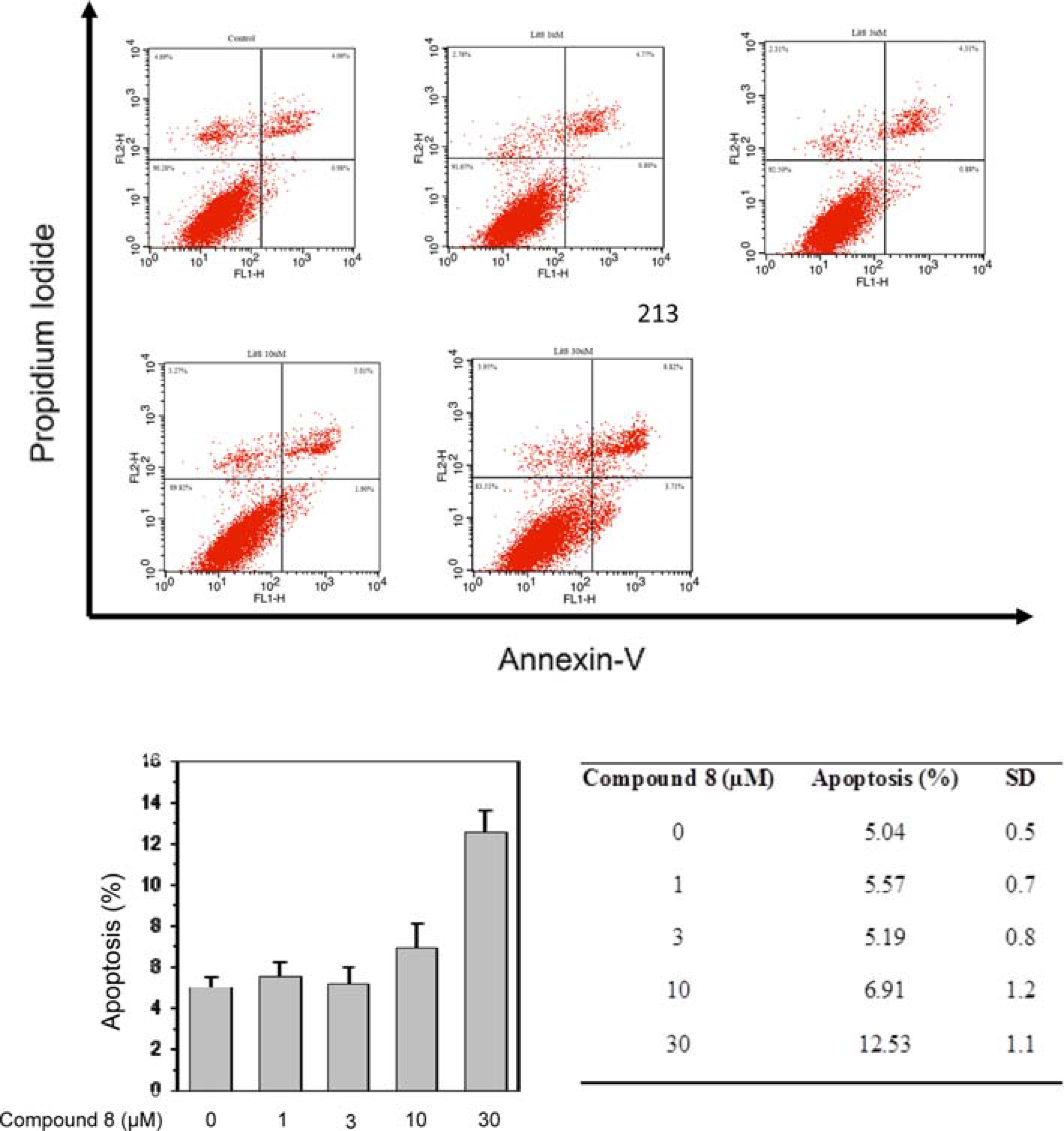
- From the pericarps of Litsea japonica (Thunb.) Jussieu, eighteen butanolide derivatives (1 - 18) were evaluated for their cytotoxic activity against HeLa, HL-60, and MCF-7 cells. Compounds 1 - 9 with 2-alkylidene-3-hydroxy-4-methylbutanolides structure exhibited cytotoxic activities against cancer-cell lines. Among them, compound 8 (litsenolide Dâ‚‚) exhibited the most potent cytotoxicity against the tested cell lines, including HeLa, HL-60, and MCF-7, with ICâ‚…â‚€ values of 17.6 ± 1.3, 4.2 ± 0.2, and 12.8 ± 0.0 µM, respectively. Compound 8 induced apoptosis in a dose-dependent manner. Annexin V/Propidium Iodide (PI) double staining confirmed that 8 effectively induced apoptosis in MCF-7 cells. To the best of our knowledge, we have reported cytotoxic activity of butanolides from L. japonica against these cancer-cell lines for the first time.
Keyword
Figure
Reference
-
(1). Wang Y. S., Wen Z. Q., Li B. T., Zhang H. B., Yang J. H. J.Ethnopharmacol. 2016; 181:66–107.(2). Lee S. S., Lin Y. J., Chen C. K., Liu K. C. S., Chen C. H. J.Nat. Prod. 1993; 56:1971–1976.(3). Dong S., Tong X., Li J., Huang C., Hu C., Jiao H., Gu Y.Neural. Regen. Res. 2013; 8:3193–3202.(4). Yang Y., Jiang J., Qimei L., Yan X., Zhao J., Yuan H., Qin Z., Wang M.Molecules. 2010; 15:7075–7082.(5). Wang L., Zhao J. F., Zeng X. H., Xie M. J., Yang X. D., Zhang H. B., Li L. J.Asian Nat. Prod. Res. 2009; 11:1028–1031.(6). Tsai I. L., Jeng Y. F., Duh C. Y., Chen I. S. J.Chin. Pharm. Sci. 2001; 53:291–301.(7). Min B. S., Lee S. Y., Kim J. H., Kwon O. K., Park B. Y., An R. B., Lee J. K., Moon H. I., Kim T. J., Kim Y. H., Joung H., Lee H. K. J.Nat. Prod. 2003; 66:1388–1390.(8). Chen I. S., Lai-Yaun I. L., Duh C. Y., Tsai I. L.Phytochemistry. 1998; 49:745–750.(9). Cheng H. I., Lin W. Y., Duh C. Y., Lee K. H., Tsai I. L., Chen I. S. J.Nat. Prod. 2001; 64:1502–1505.(10). Tanaka H., Takaya Y., Toyoda J., Yasuda T., Sato M., Murata J., Murata H., Kaburagi K., Iida O., Sugimura K., Sakai E.Phytochem. Lett. 2015; 11:32–36.(11). Ngo Q. T., Cao T. Q., Tran P. L., Kim J. A., Seo S. T., Kim J. C., Woo M. H., Lee J. H., Min B. S.Bioorg. Med. Chem. Lett. 2018; 28:2109–2115.(12). Guon T. E., Chung H. S.Nat. Prod. Sci. 2017; 23:227–234.(13). Taher M., Aminuddin A., Susanti D., Aminudin N. I., On S., Ahmad F., Hamidon H.Nat. Prod. Sci. 2016; 22:122–128.(14). Takeda K., Sakurawi K., Ishii H.Tetrahedron. 1972; 28:3757–3766.(15). Zhao Y., Guo Y. W., Zhang W.Helv. Chim. Acta. 2005; 88:349–353.(16). Tanaka H., Nakamura T., Ichino K., Ito K., Tanaka T.Phytochemistry. 1990; 29:857–859.(17). Ham Y. M., Ko Y. J., Song S. M., Kim J., Kim K. N., Yun J. H., Cho J. H., Ahn G., Yoon W. J. J.Funct. Foods. 2015; 13:80–88.(18). Cao H. Q., Lee B. M., Jung Y. W., Nguyen V. T., Kim J. A., Min B. S.Nat. Prod. Commun. 2017; 12:259–260.(19). Shin M., Lee B. M., Kim O., Tran H. N. K., Lee S., Hwangbo C., Min B. S., Lee J. H.Food Funct. 2018; 9:3895–3905.(20). Bold R. J., Termuhlen P. M., McConkey D. J.Surg. Oncol. 1997; 6:133–142.(21). Yang H. L., Chen C. S., Chang W. H., Lu F. J., Lai Y. C., Chen C. C., Hseu T. H., Kuo C. T., Hseu Y. C.Cancer Lett. 2006; 231:215–227.(22). Kamesaki H.Int. J. Hematol. 1998; 68:29–43.
Article(23). Park J. H., Noh T. H., Wang H., Kim N. D., Jung J. H.Nat. Prod. Sci. 2015; 21:282–288.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Occurrence of Gray Mold Caused by Botrytis cinerea on Cryptotaenia japonica in Korea
- New Meroterpenoids from a Penicillium sp. Fungus
- Unraveling Stereochemical Structure-Activity Relationships of Sesquiterpene Lactones for Inhibitory Effects on STAT3 Activation
- Immunomodulatory Effects of Callophyllis japonica Ethanol Extract on Dendritic Cells
- Responses of the Detrusor Muscle Strips of the Amyda Japonica and the Rabbit to some Autonomic Drugs