Dement Neurocogn Disord.
2018 Sep;17(3):73-82. 10.12779/dnd.2018.17.3.73.
Characteristics of Cerebral Microbleeds
- Affiliations
-
- 1Department of Neurology, Chungnam National University Hospital, Chungnam National University School of Medicine, Daejeon, Korea. aelee@cnu.ac.kr
- KMID: 2442796
- DOI: http://doi.org/10.12779/dnd.2018.17.3.73
Abstract
- Cerebral microbleeds (CMBs) are increasingly recognized neuroimaging findings, occurring with cerebrovascular disease, dementia, and aging. CMBs are associated with subsequent hemorrhagic and ischemic stroke, and also with an increased risk of cognitive deterioration and dementia. They occur in the setting of impaired small vessel integrity due to hypertension or cerebral amyloid angiopathy. This review summarizes the concepts, cause or risk factors, histopathological mechanisms, and clinical consequences of CMBs.
Keyword
MeSH Terms
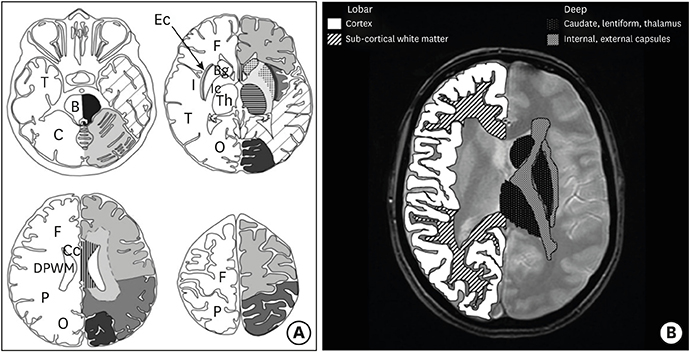
Figure
Reference
-
1. Roob G, Schmidt R, Kapeller P, Lechner A, Hartung HP, Fazekas F. MRI evidence of past cerebral microbleeds in a healthy elderly population. Neurology. 1999; 52:991–994.
Article2. Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009; 8:165–174.
Article3. Poels MM, Ikram MA, van der Lugt A, Hofman A, Krestin GP, Breteler MM, et al. Incidence of cerebral microbleeds in the general population: the Rotterdam Scan Study. Stroke. 2011; 42:656–661.
Article4. Sveinbjornsdottir S, Sigurdsson S, Aspelund T, Kjartansson O, Eiriksdottir G, Valtysdottir B, et al. Cerebral microbleeds in the population based AGES-Reykjavik study: prevalence and location. J Neurol Neurosurg Psychiatry. 2008; 79:1002–1006.
Article5. Vernooij MW, van der Lugt A, Ikram MA, Wielopolski PA, Niessen WJ, Hofman A, et al. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology. 2008; 70:1208–1214.
Article6. Poels MM, Vernooij MW, Ikram MA, Hofman A, Krestin GP, van der Lugt A, et al. Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam scan study. Stroke. 2010; 41:S103–S106.
Article7. Akoudad S, Wolters FJ, Viswanathan A, de Bruijn RF, van der Lugt A, Hofman A, et al. Association of cerebral microbleeds with cognitive decline and dementia. JAMA Neurol. 2016; 73:934–943.
Article8. Seo SW, Lee BH, Kim EJ, Chin J, Cho YS, Yoon U, et al. Clinical significance of microbleeds in subcortical vascular dementia. Stroke. 2007; 38:1949–1951.
Article9. Cordonnier C, van der Flier WM. Brain microbleeds and Alzheimer's disease: innocent observation or key player? Brain. 2011; 134:335–344.
Article10. Koennecke HC. Cerebral microbleeds on MRI: prevalence, associations, and potential clinical implications. Neurology. 2006; 66:165–171.
Article11. Lovelock CE, Cordonnier C, Naka H, Al-Shahi Salman R, Sudlow CL, et al. Edinburgh Stroke Study Group. Antithrombotic drug use, cerebral microbleeds, and intracerebral hemorrhage: a systematic review of published and unpublished studies. Stroke. 2010; 41:1222–1228.
Article12. Wilson D, Charidimou A, Ambler G, Fox ZV, Gregoire S, Rayson P, et al. Recurrent stroke risk and cerebral microbleed burden in ischemic stroke and TIA: a meta-analysis. Neurology. 2016; 87:1501–1510.
Article13. Park MY, Park HJ, Shin DS. Distribution analysis of cerebral microbleeds in Alzheimer's disease and cerebral infarction with susceptibility weighted MR imaging. J Korean Neurol Assoc. 2017; 35:72–79.
Article14. Akoudad S, Ikram MA, Koudstaal PJ, Hofman A, van der Lugt A, Vernooij MW. Cerebral microbleeds and the risk of mortality in the general population. Eur J Epidemiol. 2013; 28:815–821.
Article15. Peng Q, Sun W, Liu W, Liu R, Huang Y. CASISP Study Group. Longitudinal relationship between chronic kidney disease and distribution of cerebral microbleeds in patients with ischemic stroke. J Neurol Sci. 2016; 362:1–6.
Article16. Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013; 12:822–838.
Article17. Shoamanesh A, Kwok CS, Benavente O. Cerebral microbleeds: histopathological correlation of neuroimaging. Cerebrovasc Dis. 2011; 32:528–534.
Article18. Al-Shahi Salman R, Berg MJ, Morrison L, Awad IA. Angioma Alliance Scientific Advisory Board. Hemorrhage from cavernous malformations of the brain: definition and reporting standards. Stroke. 2008; 39:3222–3230.
Article19. Gaviani P, Mullins ME, Braga TA, Hedley-Whyte ET, Halpern EF, Schaefer PS, et al. Improved detection of metastatic melanoma by T2*-weighted imaging. AJNR Am J Neuroradiol. 2006; 27:605–608.20. Nandigam RN, Viswanathan A, Delgado P, Skehan ME, Smith EE, Rosand J, et al. MR imaging detection of cerebral microbleeds: effect of susceptibility-weighted imaging, section thickness, and field strength. AJNR Am J Neuroradiol. 2009; 30:338–343.
Article21. Haller S, Vernooij MW, Kuijer JP, Larsson EM, Jäger HR, Barkhof F. Cerebral microbleeds: imaging and clinical significance. Radiology. 2018; 287:11–28.
Article22. Haacke EM, DelProposto ZS, Chaturvedi S, Sehgal V, Tenzer M, Neelavalli J, et al. Imaging cerebral amyloid angiopathy with susceptibility-weighted imaging. AJNR Am J Neuroradiol. 2007; 28:316–317.23. Cheng AL, Batool S, McCreary CR, Lauzon ML, Frayne R, Goyal M, et al. Susceptibility-weighted imaging is more reliable than T2*-weighted gradient-recalled echo MRI for detecting microbleeds. Stroke. 2013; 44:2782–2786.
Article24. Seghier ML, Kolanko MA, Leff AP, Jäger HR, Gregoire SM, Werring DJ. Microbleed detection using automated segmentation (MIDAS): a new method applicable to standard clinical MR images. PLoS One. 2011; 6:e17547.
Article25. Kuijf HJ, Brundel M, de Bresser J, van Veluw SJ, Heringa SM, Viergever MA, et al. Semi-automated detection of cerebral microbleeds on 3.0 T MR images. PLoS One. 2013; 8:e66610.
Article26. Gregoire SM, Chaudhary UJ, Brown MM, Yousry TA, Kallis C, Jäger HR, et al. The Microbleed Anatomical Rating Scale (MARS): reliability of a tool to map brain microbleeds. Neurology. 2009; 73:1759–1766.
Article27. Cordonnier C, Potter GM, Jackson CA, Doubal F, Keir S, Sudlow CL, et al. improving interrater agreement about brain microbleeds: development of the Brain Observer MicroBleed Scale (BOMBS). Stroke. 2009; 40:94–99.
Article28. Qiu C, Ding J, Sigurdsson S, Fisher DE, Zhang Q, Eiriksdottir G, et al. Differential associations between retinal signs and CMBs by location: the AGES-Reykjavik Study. Neurology. 2018; 90:e142–e148.29. Granger JP. An emerging role for inflammatory cytokines in hypertension. Am J Physiol Heart Circ Physiol. 2006; 290:H923–H924.
Article30. Shoamanesh A, Preis SR, Beiser AS, Vasan RS, Benjamin EJ, Kase CS, et al. Inflammatory biomarkers, cerebral microbleeds, and small vessel disease: Framingham Heart Study. Neurology. 2015; 84:825–832.
Article31. Fisher M. Cerebral microbleeds and thrombolysis: clinical consequences and mechanistic implications. JAMA Neurol. 2016; 73:632–635.32. Jeon SB, Kang DW, Cho AH, Lee EM, Choi CG, Kwon SU, et al. Initial microbleeds at MR imaging can predict recurrent intracerebral hemorrhage. J Neurol. 2007; 254:508–512.
Article33. Vergouwen MD, de Haan RJ, Vermeulen M, Roos YB. Statin treatment and the occurrence of hemorrhagic stroke in patients with a history of cerebrovascular disease. Stroke. 2008; 39:497–502.
Article34. Hackam DG, Woodward M, Newby LK, Bhatt DL, Shao M, Smith EE, et al. Statins and intracerebral hemorrhage: collaborative systematic review and meta-analysis. Circulation. 2011; 124:2233–2242.35. Oh MY, Lee H, Kim JS, Ryu WS, Lee SH, Ko SB, et al. Cystatin C, a novel indicator of renal function, reflects severity of cerebral microbleeds. BMC Neurol. 2014; 14:127.
Article36. Yates PA, Villemagne VL, Ellis KA, Desmond PM, Masters CL, Rowe CC. Cerebral microbleeds: a review of clinical, genetic, and neuroimaging associations. Front Neurol. 2014; 4:205.
Article37. Lee SH, Lee ST, Kim BJ, Park HK, Kim CK, Jung KH, et al. Dynamic temporal change of cerebral microbleeds: long-term follow-up MRI study. PLoS One. 2011; 6:e25930.
Article38. McCarron MO, Nicoll JA, Stewart J, Ironside JW, Mann DM, Love S, et al. The apolipoprotein E ε2 allele and the pathological features in cerebral amyloid angiopathy-related hemorrhage. J Neuropathol Exp Neurol. 1999; 58:711–718.
Article39. Greenberg SM, Nandigam RN, Delgado P, Betensky RA, Rosand J, Viswanathan A, et al. Microbleeds versus macrobleeds: evidence for distinct entities. Stroke. 2009; 40:2382–2386.40. Wang Z, Soo YO, Mok VC. Cerebral microbleeds: is antithrombotic therapy safe to administer? Stroke. 2014; 45:2811–2817.41. Pasi M, Charidimou A, Boulouis G, Auriel E, Ayres A, Schwab KM, et al. Mixed-location cerebral hemorrhage/microbleeds: underlying microangiopathy and recurrence risk. Neurology. 2018; 90:e119–e126.42. Charidimou A, Boulouis G, Pasi M, Auriel E, van Etten ES, Haley K, et al. MRI-visible perivascular spaces in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology. 2017; 88:1157–1164.
Article43. Martinez-Ramirez S, Pontes-Neto OM, Dumas AP, Auriel E, Halpin A, Quimby M, et al. Topography of dilated perivascular spaces in subjects from a memory clinic cohort. Neurology. 2013; 80:1551–1556.
Article44. Renard D. Cerebral microbleeds: a magnetic resonance imaging review of common and less common causes. Eur J Neurol. 2018; 25:441–450.
Article45. Unetani H, Hirai T, Hashimoto M, Ikeda M, Kitajima M, Sakamoto F, et al. Prevalence and topography of small hypointense foci suggesting microbleeds on 3T susceptibility-weighted imaging in various types of dementia. AJNR Am J Neuroradiol. 2013; 34:984–989.
Article46. Kato H, Izumiyama M, Izumiyama K, Takahashi A, Itoyama Y. Silent cerebral microbleeds on T2*-weighted MRI: correlation with stroke subtype, stroke recurrence, and leukoaraiosis. Stroke. 2002; 33:1536–1540.47. Imaizumi T, Horita Y, Hashimoto Y, Niwa J. Dotlike hemosiderin spots on T2*-weighted magnetic resonance imaging as a predictor of stroke recurrence: a prospective study. J Neurosurg. 2004; 101:915–920.
Article48. Bokura H, Saika R, Yamaguchi T, Nagai A, Oguro H, Kobayashi S, et al. Microbleeds are associated with subsequent hemorrhagic and ischemic stroke in healthy elderly individuals. Stroke. 2011; 42:1867–1871.
Article49. van Etten ES, Auriel E, Haley KE, Ayres AM, Vashkevich A, Schwab KM, et al. Incidence of symptomatic hemorrhage in patients with lobar microbleeds. Stroke. 2014; 45:2280–2285.
Article50. Soo YO, Yang SR, Lam WW, Wong A, Fan YH, Leung HH, et al. Risk vs benefit of anti-thrombotic therapy in ischaemic stroke patients with cerebral microbleeds. J Neurol. 2008; 255:1679–1686.
Article51. Altmann-Schneider I, Trompet S, de Craen AJ, van Es AC, Jukema JW, Stott DJ, et al. Cerebral microbleeds are predictive of mortality in the elderly. Stroke. 2011; 42:638–644.
Article52. Zhu YC, Chabriat H, Godin O, Dufouil C, Rosand J, Greenberg SM, et al. Distribution of white matter hyperintensity in cerebral hemorrhage and healthy aging. J Neurol. 2012; 259:530–536.
Article53. Liu W, Liu R, Sun W, Peng Q, Zhang W, Xu E, et al. Different impacts of blood pressure variability on the progression of cerebral microbleeds and white matter lesions. Stroke. 2012; 43:2916–2922.
Article54. Schuur M, van Swieten JC, Schol-Gelok S, Ikram MA, Vernooij MW, Liu F, et al. Genetic risk factors for cerebral small-vessel disease in hypertensive patients from a genetically isolated population. J Neurol Neurosurg Psychiatry. 2011; 82:41–44.
Article55. Selim M, Diener HC. Atrial fibrillation and microbleeds. Stroke. 2017; 48:2660–2664.
Article56. Greenberg SM, Bacskai BJ, Hyman BT. Alzheimer disease's double-edged vaccine. Nat Med. 2003; 9:389–390.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Relationship Between Cerebral Microbleeds and Aspirin Use Regarding White Matter Hyperintensity Volume
- Cerebral Microbleed Induced Seizure Misdiagnosed with Transient Ischemic Attack
- Diffuse cerebral microbleeds in a young adult with Down syndrome
- Microbleeds in the Corpus Callosum in Anoxic Brain Injury
- Silent Microbleeds and Hemorrhagic Conversion of an Embolic Infarction