Nutr Res Pract.
2018 Jun;12(3):183-190. 10.4162/nrp.2018.12.3.183.
A Portulaca oleracea L. extract promotes insulin secretion via a Kâº(ATP) channel dependent pathway in INS-1 pancreatic β-cells
- Affiliations
-
- 1Department of Food Science and Nutrition, Pusan National University, 2, Busandaehak-ro 63beon-gil, Geumjeong-gu, Busan 46241, Korea. hanjs@pusan.ac.kr
- KMID: 2442291
- DOI: http://doi.org/10.4162/nrp.2018.12.3.183
Abstract
- BACKGROUND
/OBJECTIVE: This study was designed to investigate how a Portulaca oleracea L. extract (POE) stimulates insulin secretion in INS-1 pancreatic β-cells. MATERIALS/METHOD: INS-1 pancreatic β-cells were incubated in the presence of various glucose concentrations: 1.1 or 5.6, 16.7 mM glucose. The cells were treated with insulin secretagogues or insulin secretion inhibitor for insulin secretion assay using an insulin ELISA kit. In order to quantify intracellular influx of Ca2+ caused by POE treatment, the effect of POE on intracellular Ca2+ in INS-1 pancreatic β-cells was examined using Fluo-2 AM dye.
RESULTS
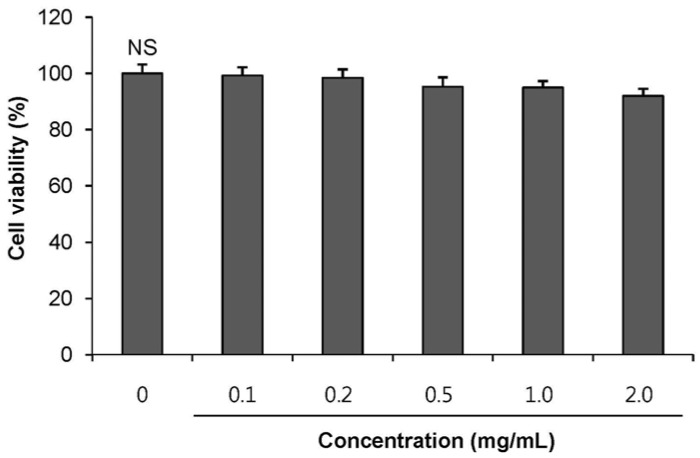
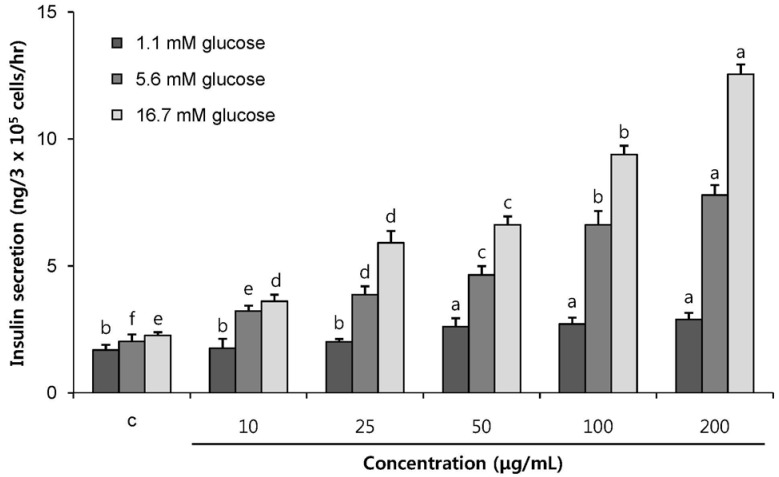
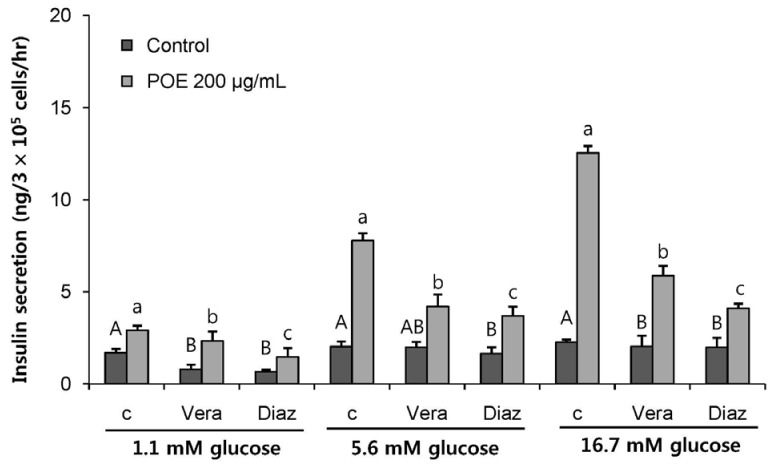
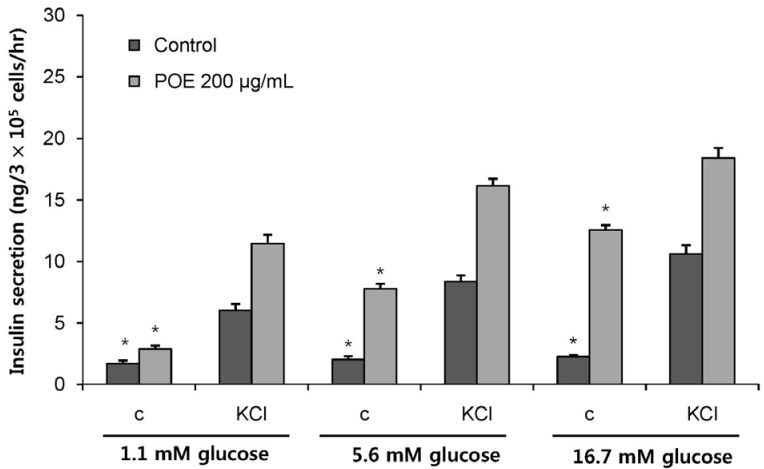
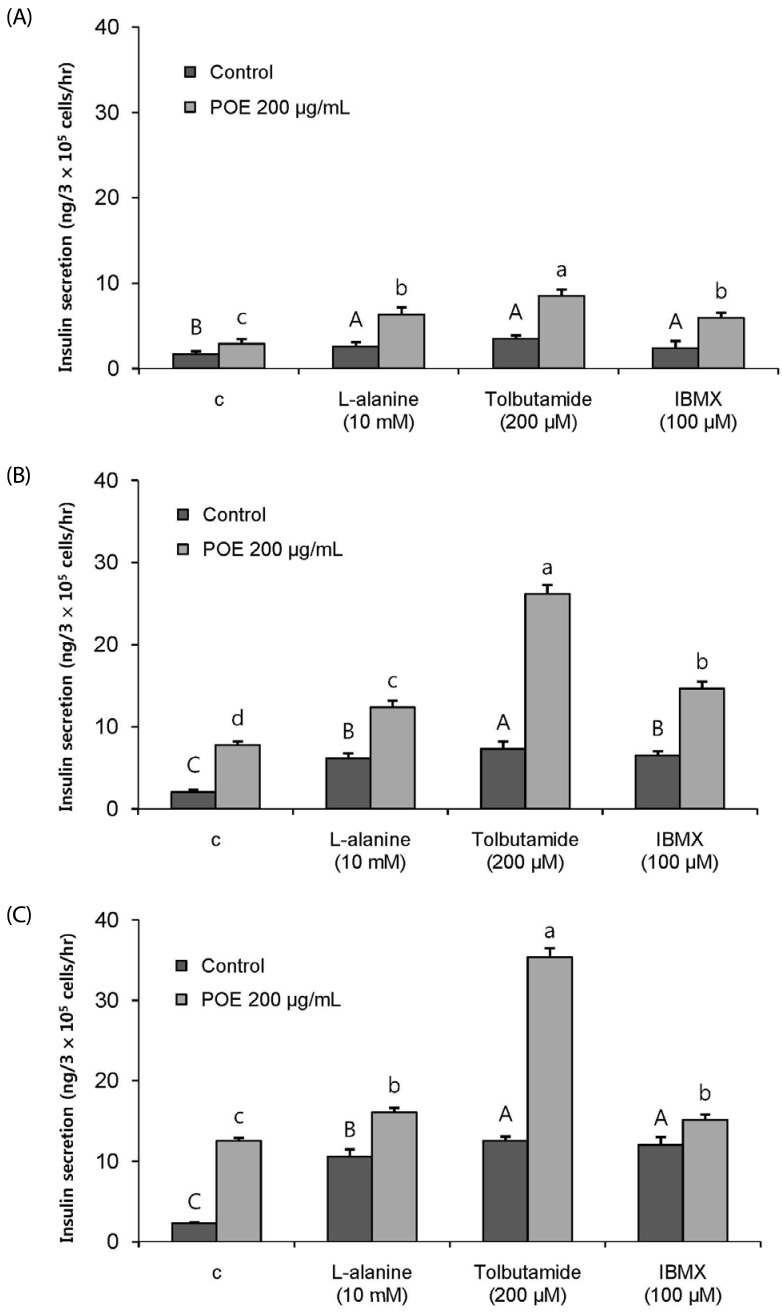
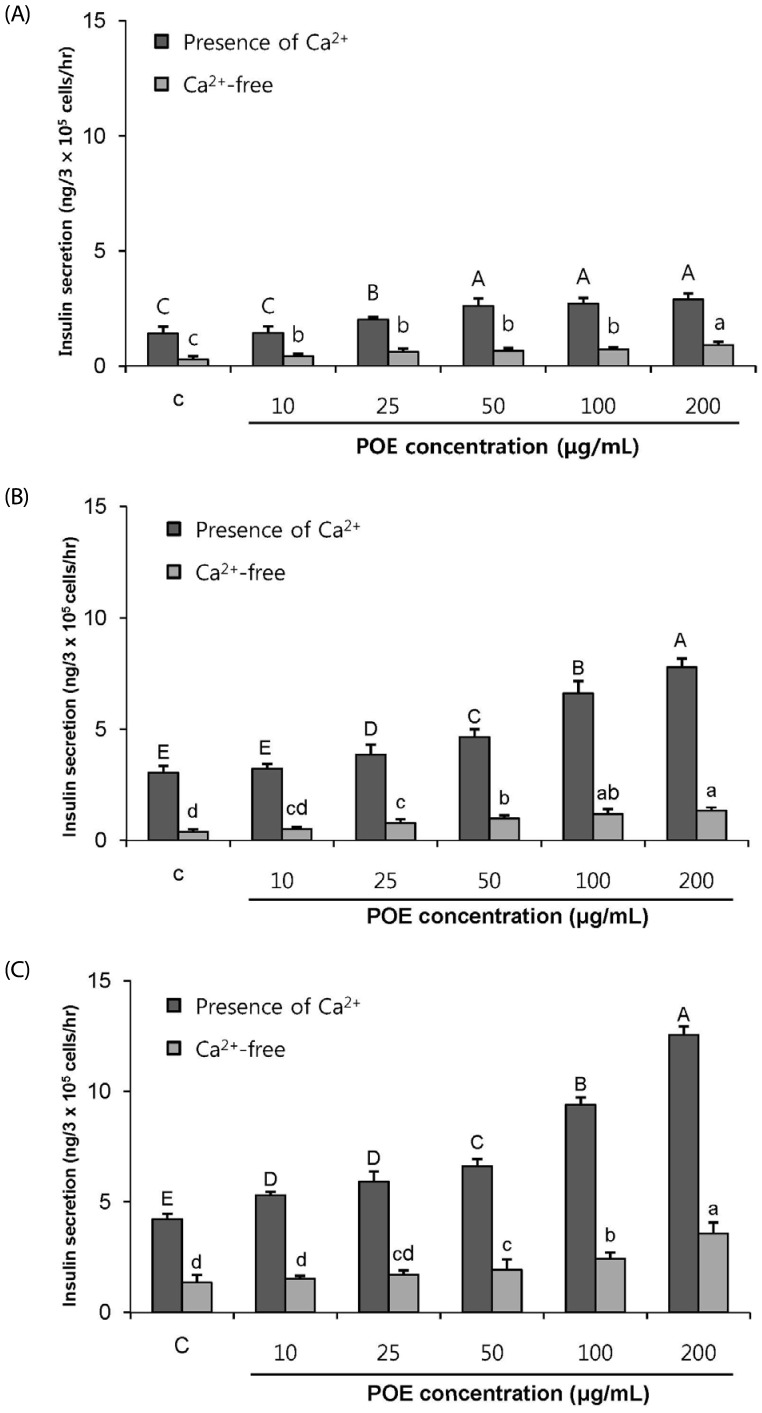
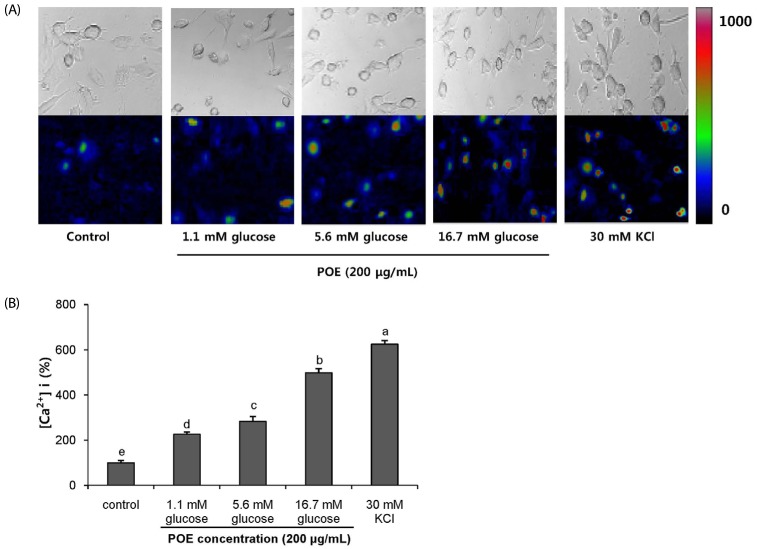
POE at 10 to 200 µg/mL significantly increased insulin secretion dose-dependently as compared to the control. Experiments at three glucose concentrations (1.1, 5.6, and 16.7 mM) confirmed that POE significantly stimulated insulin secretion on its own as well as in a glucose-dependent manner. POE also exerted synergistic effects on insulin secretion with secretagogues, such as L-alanine, 3-isobutyl-1-methylxanthine, and especially tolbutamide, and at a depolarizing concentration of KCl. The insulin secretion caused by POE was significantly attenuated by treatment with diazoxide, an opener of the K+ ATP channel (blocking insulin secretion) and by verapamil (a Ca2+ channel blocker). The insulinotropic effect of POE was not observed under Ca2+-free conditions in INS-1 pancreatic β-cells. When the cells were preincubated with a Ca2+ fluorescent dye, Fluo-2 (acetoxymethyl ester), the cells treated with POE showed changes in fluorescence in red, green, and blue tones, indicating a significant increase in intracellular Ca2+, which closely correlated with increases in the levels of insulin secretion.
CONCLUSIONS
These findings indicate that POE stimulates insulin secretion via a K+ ATP channel-dependent pathway in INS-1 pancreatic β-cells.
Keyword
MeSH Terms
-
1-Methyl-3-isobutylxanthine
Adenosine Triphosphate
Alanine
Calcium Channels
Diabetes Mellitus
Diazoxide
Enzyme-Linked Immunosorbent Assay
Fluorescence
Glucose
Insulin*
Portulaca*
Tolbutamide
Verapamil
1-Methyl-3-isobutylxanthine
Adenosine Triphosphate
Alanine
Calcium Channels
Diazoxide
Glucose
Insulin
Tolbutamide
Verapamil
Figure
Reference
-
1. Beardsall K, Yuen K, Williams R, Dunger D. Applied physiology glucose control. Curr Paediatr. 2003; 13:543–548.2. Ferrer JC, Favre C, Gomis RR, Fernández-Novell JM, Garcia-Rocha M, de la Iglesia N, Cid E, Guinovart JJ. Control of glycogen deposition. FEBS Lett. 2003; 546:127–132. PMID: 12829248.
Article3. Düfer M, Haspel D, Krippeit-Drews P, Kelm M, Ranta F, Nitschke R, Ullrich S, Aguilar-Bryan L, Bryan J, Drews G. The KATP channel is critical for calcium sequestration into non-ER compartments in mouse pancreatic beta cells. Cell Physiol Biochem. 2007; 20:65–74. PMID: 17595516.
Article4. Tanaka T, Nagashima K, Inagaki N, Kioka H, Takashima S, Fukuoka H, Noji H, Kakizuka A, Imamura H. Glucose-stimulated single pancreatic islets sustain increased cytosolic ATP levels during initial Ca2+ influx and subsequent Ca2+ oscillations. J Biol Chem. 2014; 289:2205–2216. PMID: 24302735.5. Ratner RE. Controlling postprandial hyperglycemia. Am J Cardiol. 2001; 88:26H–31H.
Article6. Henquin JC. Pathways in β-cell stimulus-secretion coupling as targets for therapeutic insulin secretagogues. Diabetes. 2004; 53(Suppl 3):S48–S58. PMID: 15561921.7. Del Prato S, Pulizzi N. The place of sulfonylureas in the therapy for type 2 diabetes mellitus. Metabolism. 2006; 55:S20–S27.
Article8. Modak M, Dixit P, Londhe J, Ghaskadbi S, Devasagayam TPA. Indian herbs and herbal drugs for the treatment of diabetes. J Clin Biochem Nutr. 2007; 40:163–173. PMID: 18398493.9. Hasani-Ranjbar S, Larijani B, Abdollahi M. A systematic review of Iranian medicinal plants useful in diabetes mellitus. Arch Med Sci. 2008; 4:285–292.10. Liu XF, Zheng CG, Shi HG, Tang GS, Wang WY, Zhou J, Dong LW. Ethanol extract from Portulaca oleracea L. attenuated acetaminophen-induced mice liver injury. Am J Transl Res. 2015; 7:309–318. PMID: 25901199.11. Zhou YX, Xin HL, Rahman K, Wang SJ, Peng C, Zhang H. Portulaca oleracea L.: a review of phytochemistry and pharmacological effects. BioMed Res Int. 2015; 2015:925631. PMID: 25692148.12. Lee AS, Lee YJ, Lee SM, Yoon JJ, Kim JS, Kang DG, Lee HS. Portulaca oleracea ameliorates diabetic vascular inflammation and endothelial dysfunction in db/db mice. Evid Based Complement Alternat Med. 2012; 2012:741824. PMID: 22474522.13. Xu X, Yu L, Chen G. Determination of flavonoids in Portulaca oleracea L. by capillary electrophoresis with electrochemical detection. J Pharm Biomed Anal. 2006; 41:493–499. PMID: 16516429.
Article14. Chan K, Islam MW, Kamil M, Radhakrishnan R, Zakaria MN, Habibullah M, Attas A. The analgesic and anti-inflammatory effects of Portulaca oleracea L. subsp. sativa (Haw.) Celak. J Ethnopharmacol. 2000; 73:445–451. PMID: 11090998.
Article15. Abdel Moneim AE. The neuroprotective effects of purslane (Portulaca oleracea) on rotenone-induced biochemical changes and apoptosis in brain of rat. CNS Neurol Disord Drug Targets. 2013; 12:830–841. PMID: 23844694.16. Gu JF, Zheng ZY, Yuan JR, Zhao BJ, Wang CF, Zhang L, Xu QY, Yin GW, Feng L, Jia XB. Comparison on hypoglycemic and antioxidant activities of the fresh and dried Portulaca oleracea L. in insulin-resistant HepG2 cells and streptozotocin-induced C57BL/6 J diabetic mice. J Ethnopharmacol. 2015; 161:214–223. PMID: 25523372.17. Niu B, Li C, Su H, Li Q, He Q, Liu L, Xue Y, Shen T, Xia X. Glucagon-like peptide-1 receptor agonist exendin-4 protects against interleukin-1 β-mediated inhibition of glucose-stimulated insulin secretion by mouse insulinoma β cells. Exp Ther Med. 2017; 14:2671–2676. PMID: 28947919.18. Tanaka Y, Tran PO, Harmon J, Robertson RP. A role for glutathione peroxidase in protecting pancreatic beta cells against oxidative stress in a model of glucose toxicity. Proc Natl Acad Sci U S A. 2002; 99:12363–12368. PMID: 12218186.19. Kim BM, Min BH, Park IS. Transplantation of Langerhans islet into digestive organs of the diabetic rat. Korean J Anat. 1990; 32:869–881.20. Nenquin M, Szollosi A, Aguilar-Bryan L, Bryan J, Henquin JC. Both triggering and amplifying pathways contribute to fuel-induced insulin secretion in the absence of sulfonylurea receptor-1 in pancreatic β-cells. J Biol Chem. 2004; 279:32316–32324. PMID: 15175349.
Article21. Leibiger B, Moede T, Schwarz T, Brown GR, Köhler M, Leibiger IB, Berggren PO. Short-term regulation of insulin gene transcription by glucose. Proc Natl Acad Sci U S A. 1998; 95:9307–9312. PMID: 9689076.
Article22. Díaz-Flores M, Angeles-Mejia S, Baiza-Gutman LA, Medina-Navarro R, Hernández-Saavedra D, Ortega-Camarillo C, Roman-Ramos R, Cruz M, Alarcon-Aguilar FJ. Effect of an aqueous extract of Cucurbita ficifolia Bouché on the glutathioneredox cycle in mice with STZ-induced diabetes. J Ethnopharmacol. 2012; 144:101–108. PMID: 22960550.23. Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009; 32:1335–1343. PMID: 19564476.
Article24. Pirart J. Diabetes mellitus and its degenerative complieations: a prospective study of 4400 patients observed between 1947 and 1973. Diabetes Care. 1978; 1:168–188.25. Dennis JW, Laferté S, Waghorne C, Breitman ML, Kerbel RS. Beta 1-6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science. 1987; 236:582–585. PMID: 2953071.26. Turner RC, Holman RR. Lessons from UK prospective diabetes study. Diabetes Res Clin Pract. 1995; 28(Suppl):S151–S157. PMID: 8529508.
Article27. Bander A. Mechanism of action of hypoglycemic sulfonyl urea compounds, D 860 & BZ 55. Dtsch Med Wochenschr. 1959; 84:996–1002. PMID: 13652690.28. Assad T, Khan RA, Feroz Z. Evaluation of hypoglycemic and hypolipidemic activity of methanol extract of Brassica oleracea. Chin J Nat Med. 2014; 12:648–653. PMID: 25263975.
Article29. El-Sayed MI. Effects of Portulaca oleracea L. seeds in treatment of type-2 diabetes mellitus patients as adjunctive and alternative therapy. J Ethnopharmacol. 2011; 137:643–651. PMID: 21718775.
Article30. Movahedian A, Ghannadi A, Vashirnia M. Hypocholesterolemic effects of purslane extracts on serum lipids in rabbits fed with high cholesterol levels. Int J Pharmacol. 2007; 3:285–289.31. Shehata MMSM, Soltan SSA. The effects of purslane and celery on hypercholesterolemic mice. World J Diary Food Sci. 2012; 7:212–221.32. Karimi G, Khoei A, Omidi A, Kalantari M, Babaei J, Taghiabadi E, Razavi BM. Protective effect of aqueous and ethanolic extracts of Portulaca oleracea against cisplatin induced nephrotoxicity. Iran J Basic Med Sci. 2010; 13:31–35.33. Hozayen W, Bastawy M, Elshafeey H. Effects of aqueous purslane (Portulaca oleracea) extract and fish oil on gentamicin nephrotoxicity in albino rats. Nat Sci. 2011; 9:47–62.34. Lan S, Fu-er L. Effects of Portulaca oleracea on insulin resistance in rats with type 2 diabetes mellitus. Chin J Integr Med. 2003; 9:289–292.
Article35. Kimura M, Waki I, Chujo T, Kikuchi T, Hiyama C, Yamazaki K, Tanaka O. Effects of hypoglycemic components in ginseng radix on blood insulin level in alloxan diabetic mice and on insulin release from perfused rat pancreas. J Pharmacobiodyn. 1981; 4:410–417. PMID: 7026762.
Article36. Kim K, Kim HY. Korean red ginseng stimulates insulin release from isolated rat pancreatic islets. J Ethnopharmacol. 2008; 120:190–195. PMID: 18773949.37. Patil SB, Dongare VR, Kulkarni CR, Joglekar MM, Arvindekar AU. Antidiabetic activity of Kalanchoe pinnata in streptozotocin-induced diabetic rats by glucose independent insulin secretagogue action. Pharm Biol. 2013; 51:1411–1418. PMID: 23865837.38. Trube G, Rorsman P, Ohno-Shosaku T. Opposite effects of tolbutamide and diazoxide on the ATP-dependent K+ channel in mouse pancreatic beta-cells. Pflugers Arch. 1986; 407:493–499. PMID: 2431383.39. Thams P, Anwar MR, Capito K. Glucose triggers protein kinase A-dependent insulin secretion in mouse pancreatic islets through activation of the K+ATP channel-dependent pathway. Eur J Endocrinol. 2005; 152:671–677. PMID: 15817925.40. Grill V, Radtke M, Qvigstad E, Kollind M, Björklund A. Beneficial effects of K-ATP channel openers in diabetes: an update on mechanisms and clinical experiences. Diabetes Obes Metab. 2009; 11(Suppl 4):143–148.
Article41. Chen WP, Chi TC, Chuang LM, Su MJ. Resveratrol enhances insulin secretion by blocking K(ATP) and K(V) channels of beta cells. Eur J Pharmacol. 2007; 568:269–277. PMID: 17573071.
Article42. Xin HL, Xu YF, Hou YH, Zhang YN, Yue XQ, Lu JC, Ling CQ. Two novel triterpenoids from Portulaca oleracea L. Helv Chim Acta. 2008; 91:2075–2080.43. Castro AJ, Cazarolli LH, de Carvalho FK, de Luz G, Altenhofen D, dos Santos AR, Pizzolatti MG, Silva FR. Acute effect of 3beta-hidroxihop-22(29)ene on insulin secretion is mediated by GLP-1, potassium and calcium channels for the glucose homeostasis. J Steroid Biochem Mol Biol. 2015; 150:112–122. PMID: 25843210.44. Hannan JM, Marenah L, Ali L, Rokeya B, Flatt PR, Abdel-Wahab YH. Ocimum sanctum leaf extracts stimulate insulin secretion from perfused pancreas, isolated islets and clonal pancreatic beta-cells. J Endocrinol. 2006; 189:127–136. PMID: 16614387.45. Guldstrand M, Grill V, Björklund A, Lins PE, Adamson U. Improved beta cell function after short-term treatment with diazoxide in obese subjects with type 2 diabetes. Diabetes Metab. 2002; 28:448–456. PMID: 12522324.46. Herchuelz A, Malaisse WJ. Regulation of calcium fluxes in pancreatic islets: association between calcium and insulin release. J Physiol. 1978; 283:409–424. PMID: 364009.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Portulaca Oleracea L. Extract on Lipolysis and Hormone Sensitive Lipase (HSL) Gene Expression in 3T3-L1 Adipocytes
- Anti-inflammatory and Anti-pruritic Effects of Portulaca oleracea L. Extract Using In Vitro and In Vivo Inflammation Model: LPS-treated Raw264.7 Cells, Keratinocytes, NC/Nga Mice and Hairless SKH-1 Mice
- Resistin Inhibits Insulin Secretion Through Inhibition of Insulin Granule Docking Via Downregulation of Rab3A in Pancreatic Beta-cells
- Effect of Intracellular ATP on Zn2+ Blockade of KATP Channels in Pancreatic Beta Cells
- Hexane Extract of Orthosiphon stamineus Induces Insulin Expression and Prevents Glucotoxicity in INS-1 Cells