J Breast Cancer.
2019 Mar;22(1):96-108. 10.4048/jbc.2019.22.e13.
Young Patients with Hormone Receptor-Positive Breast Cancer Have a Higher Long-Term Risk of Breast Cancer Specific Death
- Affiliations
-
- 1Department of Oncology, Jinhua Central Hospital, Jinhua, China.
- 2Department of Medical Oncology, The Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, China.
- 3Cancer Institute (Key Laboratory of Cancer Prevention and Intervention, Chinese National Ministry of Education; Key Laboratory of Molecular Biology in Medical Sciences, Zhejiang Province, China), The Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, China.
- 4Department of Gastroenterology, The Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, China.
- 5Institution of Gastroenterology, Zhejiang University, Hangzhou, China.
- 6Institute of Translational Medicine, School of Medicine, Zhejiang University, Hangzhou, China.
- 7Department of Nuclear Medicine, Jinhua central hospital, Jinhua, China.
- 8Department of Medical Oncology, The First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, China.
- 9Department of Colorectal Surgery, Jinhua central hospital, Jinhua, China. djl9090@163.com
- KMID: 2441855
- DOI: http://doi.org/10.4048/jbc.2019.22.e13
Abstract
- PURPOSE
Although it is widely accepted that hormone receptor (HR) status is associated with later post-diagnostic periods, a debate exists as to whether the association is independent of age. The aim of our study was to confirm the impact of HR status on later period breast cancer-specific death (LP-BCSD) and later period non-breast cancer-specific death (LP-non-BCSD) in different age subgroups.
METHODS
Surveillance, Epidemiology, and End Results databases were utilized to identify 181,108 breast cancer patients with > 5 years survival. The cumulative incidence of LP-BCSD and LP-non-BCSD was calculated using the Gray method. The subdistribution hazard ratio (SHR) of variables was estimated via the Fine and Gray proportional hazard regression model. Subgroup analyses for LP-BCSD and LP-non-BCSD were performed according to the HR status.
RESULTS
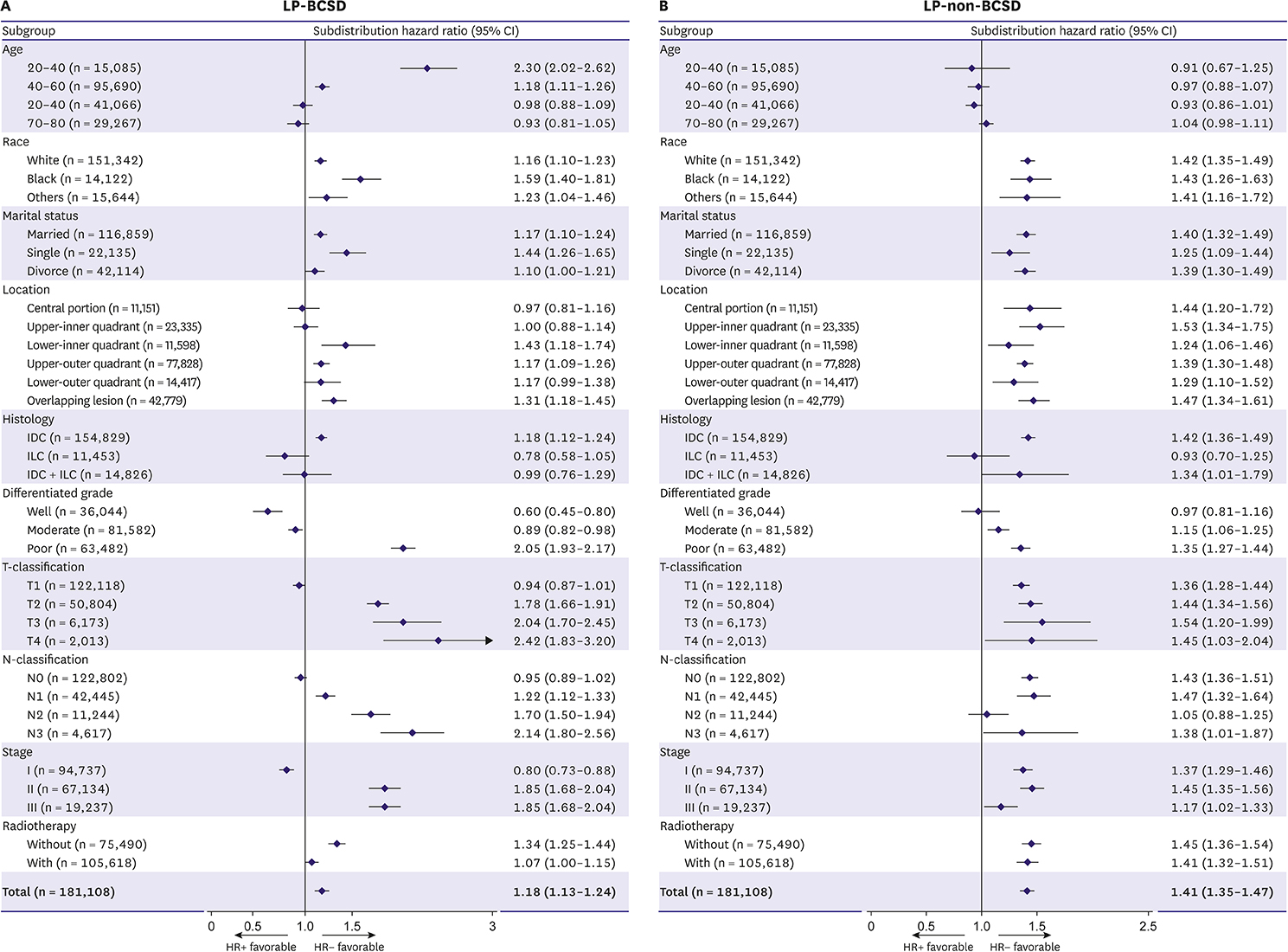
The risk of LP-BCSD was exceeded by that of LP-non-BCSD at > 5 years since the diagnosis, particularly in old women. The competing risk regression model indicated that hormone receptor-positive (HR+) was an independent factor for more LP-BCSD (hazard ratio, 1.54; 95% confidence interval, 1.44-1.54; p < 0.001). However, stratified analysis indicated that HR+ was only associated with more LP-BCSD in the young women subgroup. Although HR+ was associated with more LP-non-BCSD, the predictive value of HR+ for LP-non-BCSD was eliminated after adjusting for age.
CONCLUSIONS
HR+ was related to LP-BCSD in the premenopausal population. LP-BCSD should be an optimal endpoint in future trials designed to evaluate the role of extended adjuvant endocrine therapy.
Keyword
MeSH Terms
Figure
Reference
-
1. Copson E, Eccles B, Maishman T, Gerty S, Stanton L, Cutress RI, et al. Prospective observational study of breast cancer treatment outcomes for UK women aged 18–40 years at diagnosis: the POSH study. J Natl Cancer Inst. 2013; 105:978–988.
Article2. Hähnel R, Spilsbury K. Oestrogen receptors revisited: long-term follow up of over five thousand breast cancer patients. ANZ J Surg. 2004; 74:957–960.
Article3. Herold CI, Djulbegovic B, Hozo I, Lyman GH. Reliable data on 5- and 10-year survival provide accurate estimates of 15-year survival in estrogen receptor-positive early-stage breast cancer. Breast Cancer Res Treat. 2010; 121:771–776.
Article4. Kennecke HF, Olivotto IA, Speers C, Norris B, Chia SK, Bryce C, et al. Late risk of relapse and mortality among postmenopausal women with estrogen responsive early breast cancer after 5 years of tamoxifen. Ann Oncol. 2007; 18:45–51.
Article5. O'Leary CG, Ellis H, Higgins M. Extended adjuvant endocrine therapy in hormone-receptor-positive early breast cancer. Curr Opin Oncol. 2016; 28:455–460.6. Overmoyer B. Treatment with adjuvant endocrine therapy for early-stage breast cancer: is it forever? J Clin Oncol. 2015; 33:823–828.
Article7. Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988; 16:1140–1154.
Article8. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999; 94:496–509.
Article9. Fu J, Wu L, Jiang M, Li D, Jiang T, Fu W, et al. Real-world impact of non-breast cancer-specific death on overall survival in resectable breast cancer. Cancer. 2017; 123:2432–2443.
Article10. Ribnikar D, Ribeiro JM, Pinto D, Sousa B, Pinto AC, Gomes E, et al. Breast cancer under age 40: a different approach. Curr Treat Options Oncol. 2015; 16:16.
Article11. Christinat A, Di Lascio S, Pagani O. Hormonal therapies in young breast cancer patients: when, what and for how long? J Thorac Dis. 2013; 5:Suppl 1. S36–S46.12. Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013; 381:805–816.13. Goss PE, Ingle JN, Pritchard KI, Robert NJ, Muss H, Gralow J, et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med. 2016; 375:209–219.
Article14. Mamounas EP, Bandos H, Lembersky BC, Geyer CE Jr, Fehrenbacher L, Graham ML, et al. Abstract S1-05: a randomized, double-blinded, placebo-controlled clinical trial of extended adjuvant endocrine therapy (tx) with letrozole (L) in postmenopausal women with hormone-receptor (+) breast cancer (BC) who have completed previous adjuvant tx with an aromatase inhibitor (AI): results from NRG Oncology/NSABP B-42. In : 2016 San Antonio Breast Cancer Symposium; December 6–10, 2016; San Antonio (TX). Philadelphia (PA): American Association for Cancer Research;2017. DOI: 10.1158/1538-7445.SABCS16-S1-05.15. Mamounas EP, Lembersky B, Jeong JH, Cronin W, Harkins B, Geyer C, et al. NSABP B-42: a clinical trial to determine the efficacy of five years of letrozole compared with placebo in patients completing five years of hormonal therapy consisting of an aromatase inhibitor (AI) or tamoxifen followed by an AI in prolonging disease-free survival in postmenopausal women with hormone receptor-positive breast cancer. Clin Breast Cancer. 2006; 7:416–421.
Article16. Jatoi I, Anderson WF, Jeong JH, Redmond CK. Breast cancer adjuvant therapy: time to consider its time-dependent effects. J Clin Oncol. 2011; 29:2301–2304.
Article17. Amir E, Seruga B, Niraula S, Carlsson L, Ocaña A. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst. 2011; 103:1299–1309.
Article18. Daly B, Olopade OI, Hou N, Yao K, Winchester DJ, Huo D. Evaluation of the quality of adjuvant endocrine therapy delivery for breast cancer care in the United States. JAMA Oncol. 2017; 3:928–935.
Article19. Mariotto A, Feuer EJ, Harlan LC, Wun LM, Johnson KA, Abrams J. Trends in use of adjuvant multi-agent chemotherapy and tamoxifen for breast cancer in the United States: 1975-1999. J Natl Cancer Inst. 2002; 94:1626–1634.
Article20. Wangchinda P, Ithimakin S. Factors that predict recurrence later than 5 years after initial treatment in operable breast cancer. World J Surg Oncol. 2016; 14:223.
Article21. Nishimura R, Osako T, Nishiyama Y, Tashima R, Nakano M, Fujisue M, et al. Evaluation of factors related to late recurrence--later than 10 years after the initial treatment--in primary breast cancer. Oncology. 2013; 85:100–110.
Article22. Goss PE, Muss HB, Ingle JN, Whelan TJ, Wu M. Extended adjuvant endocrine therapy in breast cancer: current status and future directions. Clin Breast Cancer. 2008; 8:411–417.
Article23. Goldvaser H, Barnes TA, Šeruga B, Cescon DW, Ocaña A, Ribnikar D, et al. Toxicity of extended adjuvant therapy with aromatase inhibitors in early breast cancer: a systematic review and meta-analysis. J Natl Cancer Inst. 2018; 110:31–39.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hormone Treatment for Breast Cancer
- Treatment Outcomes of Weakly Positive Hormone Receptor Breast Cancer and Triple-Negative Breast Cancer
- Treatment Patterns and Outcomes of Young Female Early Breast Cancer in Taiwan
- Diagnosis and Treatment of HER2-Positive Breast Cancer
- Hormone Therapy for Metastatic Breast Cancer