Label Adherence for Non-Vitamin K Antagonist Oral Anticoagulants in a Prospective Cohort of Asian Patients with Atrial Fibrillation
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea. choiek17@snu.ac.kr
- 2Division of Cardiology, Catholic University of Daegu, Daegu, Korea.
- 3Division of Cardiology, Yonsei University College of Medicine, Seoul, Korea. cby6908@yuhs.ac
- 4Department of Cardiology, College of Medicine, Ewha Womans University, Seoul, Korea.
- 5Division of Cardiology, Hanyang University Medical Center, Seoul, Korea.
- 6Division of Cardiology, Kyung Hee University Medical College, Seoul, Korea.
- 7Division of Cardiology, Eulji University Hospital, Daejeon, Korea.
- 8Division of Cardiology, Korea University Anam Hospital, Seoul, Korea.
- 9Department of Internal Medicine, University of Ulsan College of Medicine, Seoul, Korea.
- 10Department of Preventive Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 11Department of Cardiovascular Medicine, Chonnam National University Medical School, Gwangju, Korea.
- KMID: 2441305
- DOI: http://doi.org/10.3349/ymj.2019.60.3.277
Abstract
- PURPOSE
Label adherence for non-vitamin K antagonist oral anticoagulants (NOACs) has not been well evaluated in Asian patients with non-valvular atrial fibrillation (AF). The present study aimed to assess label adherence for NOACs in a Korean AF population and to determine risk factors of off-label prescriptions of NOACs.
MATERIALS AND METHODS
In this COmparison study of Drugs for symptom control and complication prEvention of AF (CODE-AF) registry, patients with AF who were prescribed NOACs between June 2016 and May 2017 were included. Four NOAC doses were categorized as on- or off-label use according to Korea Food and Drug Regulations.
RESULTS
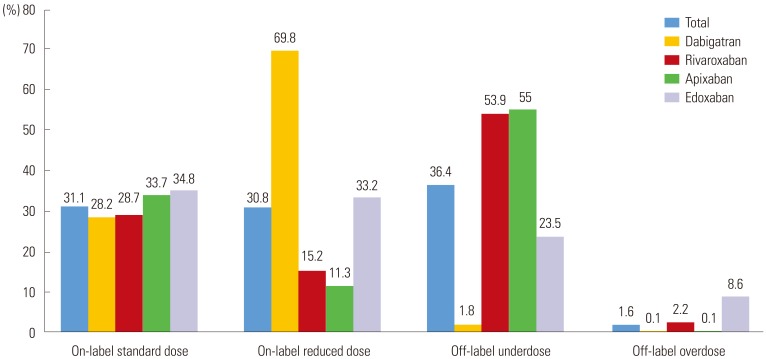
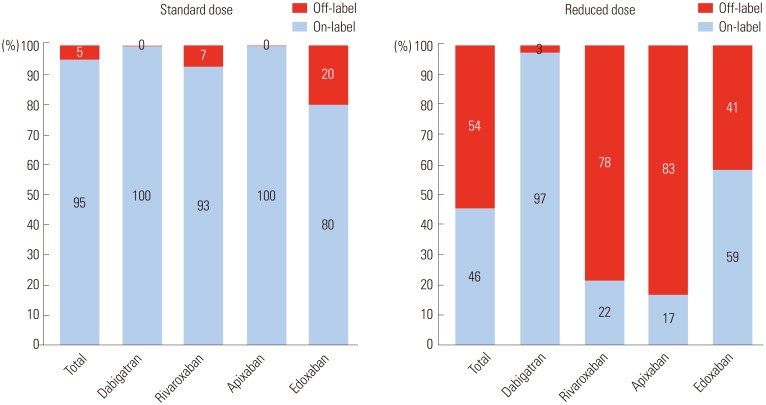
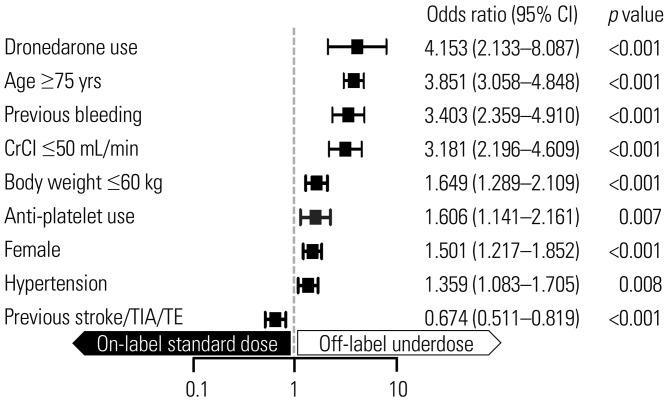
We evaluated 3080 AF patients treated with NOACs (dabigatran 27.2%, rivaroxaban 23.9%, apixaban 36.9%, and edoxaban 12.0%). The mean age was 70.5±9.2 years; 56.0% were men; and the mean CHAâ‚‚DSâ‚‚-VASc score was 3.3±1.4. Only one-third of the patients (32.7%) was prescribed a standard dose of NOAC. More than one-third of the study population (n=1122, 36.4%) was prescribed an off-label reduced dose of NOAC. Compared to those with an on-label standard dosing, patients with an off-label reduced dose of NOAC were older (≥75 years), women, and had a lower body weight (≤60 kg), renal dysfunction (creatinine clearance ≤50 mL/min), previous stroke, previous bleeding, hypertension, concomitant dronedarone use, and anti-platelet use.
CONCLUSION
In real-world practice, more than one-third of patients with NOAC prescriptions received an off-label reduced dose, which could result in an increased risk of stroke. Considering the high risk of stroke in these patients, on-label use of NOAC is recommended.
MeSH Terms
Figure
Cited by 4 articles
-
Current Anticoagulant Usage Patterns and Determinants in Korean Patients with Nonvalvular Atrial Fibrillation
Hyun Su Ha, Joongmin Kim, Young Soo Lee, Tae-Hoon Kim, Jung Myung Lee, Junbeom Park, Jin-Kyu Park, Ki-Woon Kang, Jaemin Shim, Jae-Sun Uhm, Hyung Wook Park, Myung-Jin Cha, Eue-Keun Choi, Jun Kim, Jin-Bae Kim, Changsoo Kim, Boyoung Joung
Yonsei Med J. 2020;61(2):120-128. doi: 10.3349/ymj.2020.61.2.120.Association of Gender With Clinical Outcomes in a Contemporary Cohort of Patients With Atrial Fibrillation Receiving Oral Anticoagulants
Minjeong Kim, Jun Kim, Jin-Bae Kim, Junbeom Park, Jin-Kyu Park, Ki-Woon Kang, Jaemin Shim, Eue-Keun Choi, Young Soo Lee, Hyung Wook Park, Boyoung Joung
Korean Circ J. 2022;52(8):593-603. doi: 10.4070/kcj.2021.0399.Dose Selection of Non-Vitamin K Antagonist Oral Anticoagulants in Korean Patients with Non-Valvular Atrial Fibrillation
So-Ryoung Lee, Young Keun On
Cardiovasc Prev Pharmacother. 2020;2(1):1-10. doi: 10.36011/cpp.2020.2.e5.Increasing Very Low-Dose Edoxaban Prescription: Effectiveness and Safety Data of Korean AF Patients
JungMin Choi, So-Young Yang, So-Ryoung Lee, Min Soo Cho, Kyung-Yeon Lee, Hyo-Jeong Ahn, Soonil Kwon, Myung-Jin Cha, Jun Kim, Gi-Byoung Nam, Kee-Joon Choi, Eue-Keun Choi, Seil Oh, Gregory Y. H. Lip
Korean Circ J. 2024;55(3):215-227. doi: 10.4070/kcj.2024.0222.
Reference
-
1. Ogilvie IM, Newton N, Welner SA, Cowell W, Lip GY. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med. 2010; 123:638–645. PMID: 20609686.
Article2. Huisman MV, Rothman KJ, Paquette M, Teutsch C, Diener HC, Dubner SJ, et al. The changing landscape for stroke prevention in AF: findings from the GLORIA-AF registry phase 2. J Am Coll Cardiol. 2017; 69:777–785. PMID: 28209218.3. Camm AJ, Accetta G, Ambrosio G, Atar D, Bassand JP, Berge E, et al. Evolving antithrombotic treatment patterns for patients with newly diagnosed atrial fibrillation. Heart. 2017; 103:307–314. PMID: 27647168.
Article4. Lee SR, Choi EK, Han KD, Cha MJ, Oh S, Lip GYH. Temporal trends of antithrombotic therapy for stroke prevention in Korean patients with non-valvular atrial fibrillation in the era of non-vitamin K antagonist oral anticoagulants: a nationwide population-based study. PLoS One. 2017; 12:e0189495. PMID: 29261716.
Article5. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009; 361:1139–1151. PMID: 19717844.
Article6. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011; 365:883–891. PMID: 21830957.
Article7. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011; 365:981–992. PMID: 21870978.8. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013; 369:2093–2104. PMID: 24251359.
Article9. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016; 37:2893–2962. PMID: 27567408.
Article10. Steinberg BA, Shrader P, Thomas L, Ansell J, Fonarow GC, Gersh BJ, et al. Off-label dosing of non-vitamin K antagonist oral anticoagulants and adverse outcomes: the ORBIT-AF II Registry. J Am Coll Cardiol. 2016; 68:2597–2604. PMID: 27978942.11. Yao X, Shah ND, Sangaralingham LR, Gersh BJ, Noseworthy PA. Non-vitamin K antagonist oral anticoagulant dosing in patients with atrial fibrillation and renal dysfunction. J Am Coll Cardiol. 2017; 69:2779–2790. PMID: 28595692.
Article12. Chan YH, Kuo CT, Yeh YH, Chang SH, Wu LS, Lee HF, et al. Thromboembolic, bleeding, and mortality risks of rivaroxaban and dabigatran in Asians with nonvalvular atrial fibrillation. J Am Coll Cardiol. 2016; 68:1389–1401. PMID: 27659460.13. Shen AY, Yao JF, Brar SS, Jorgensen MB, Chen W. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol. 2007; 50:309–315. PMID: 17659197.
Article14. Cha MJ, Choi EK, Han KD, Lee SR, Lim WH, Oh S, et al. Effectiveness and safety of non-vitamin K antagonist oral anticoagulants in Asian patients with atrial fibrillation. Stroke. 2017; 48:3040–3048. PMID: 28974629.
Article15. Kim H, Kim TH, Cha MJ, Lee JM, Park J, Park JK, et al. A prospective survey of atrial fibrillation management for real-world guideline adherence: COmparison study of Drugs for symptom control and complication prEvention of Atrial Fibrillation (CODE-AF) registry. Korean Circ J. 2017; 47:877–887. PMID: 29171211.
Article16. Lee KH, Park HW, Lee N, Hyun DY, Won J, Oh SS, et al. Optimal dose of dabigatran for the prevention of thromboembolism with minimal bleeding risk in Korean patients with atrial fibrillation. Europace. 2017; 19(suppl_4):iv1–iv9. PMID: 29220421.
Article17. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014; 383:955–962. PMID: 24315724.
Article18. Hori M, Matsumoto M, Tanahashi N, Momomura S, Uchiyama S, Goto S, et al. Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation - the J-ROCKET AF study. Circ J. 2012; 76:2104–2111. PMID: 22664783.