J Korean Ophthalmol Soc.
2019 Mar;60(3):239-245. 10.3341/jkos.2019.60.3.239.
Comparison of Efficacy and Sensation of Instillation between 0.05% Cyclosporine Nanoemulsion and Microemulsion Type
- Affiliations
-
- 1Department of Ophthalmology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. taeyoung15.chung@samsung.com
- 2Department of Preventive Medicine, Graduate School, The Catholic University of Korea, Seoul, Korea.
- KMID: 2440451
- DOI: http://doi.org/10.3341/jkos.2019.60.3.239
Abstract
- PURPOSE
To evaluate and compare the efficacy and sensation of instillation between 0.05% cyclosporine nanoemulsion group and microemulsion group.
METHODS
This is a double-blind, prospective randomized clinical trial. Patients had 2 weeks of wash-out period before the study. They were randomly assigned to either nanoemulsion group or microemulsion group and treated with each group's cyclosporine eye drop. Artificial eye drop and topical steroid were used together according to severity of dryness of cornea. We checked every patient's Break-up time (BUT), Schirmer test, Staining Score and Ocular surface disease index (OSDI) on baseline, 1 month and 3 months after. Patients also self-checked frequency of use of artificial eye drop and topical steroid. Sensation of instillation was also checked.
RESULTS
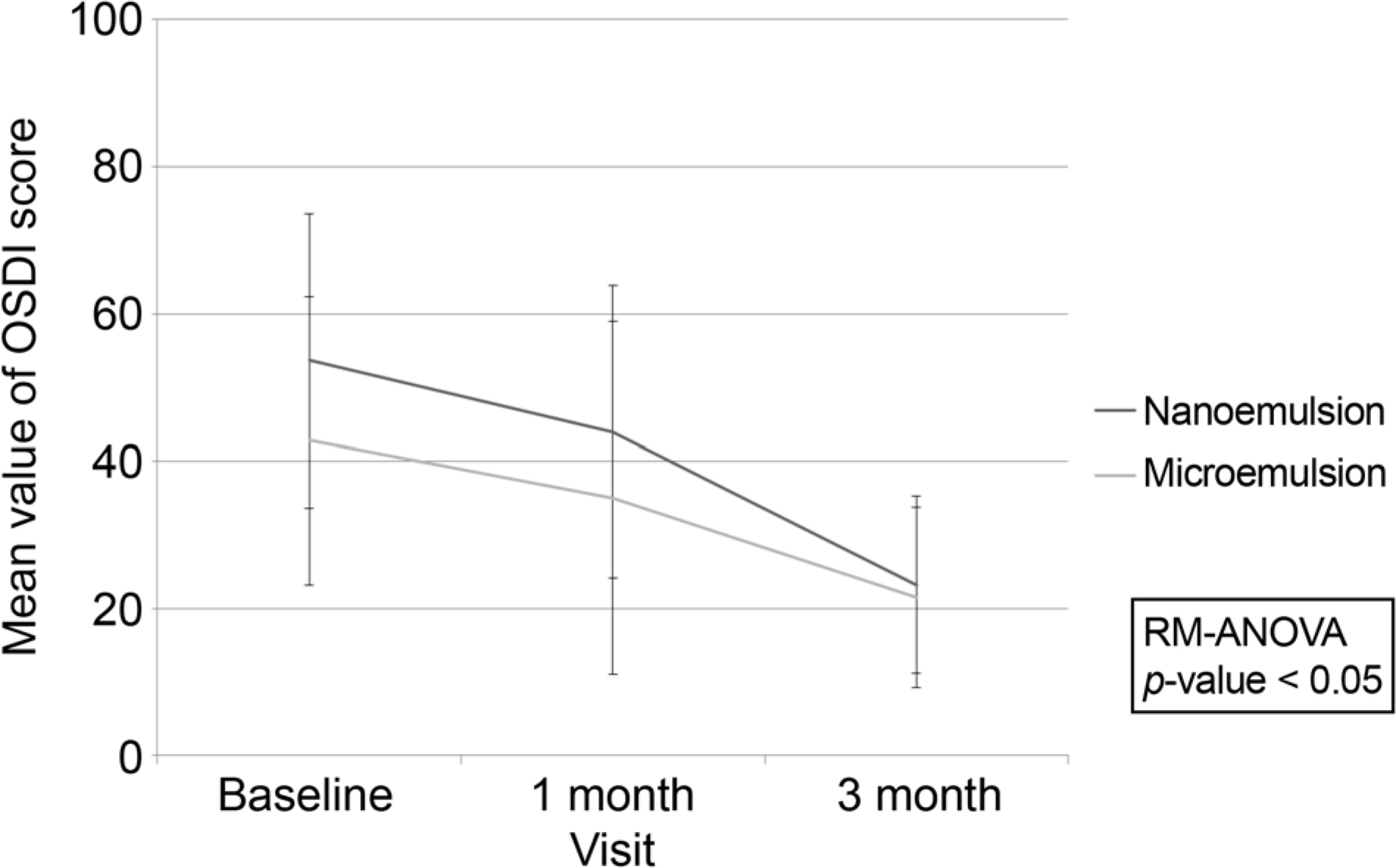
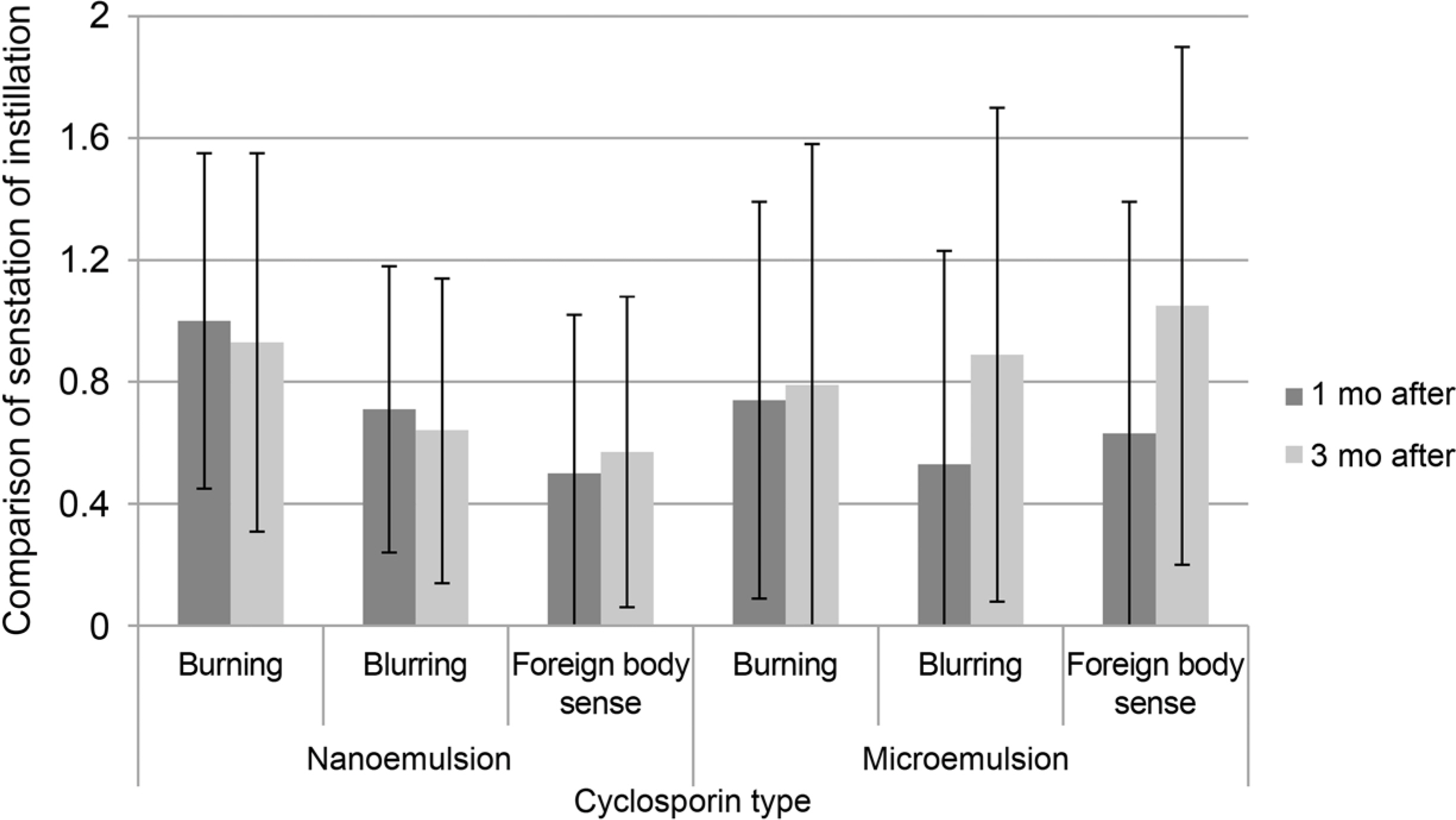
Both nanoemulsion eye-drop and microemulsion eye-drop improved BUT, Schirmer test, Staining Score and OSDI throughout 12 weeks. The nanoemulsion type reduced OSDI significantly compared to the microemulsion type. The mean frequency of use of artificial tear and topical steroid was similar in both groups. Foreign body sense score was higher in microemulsion group.
CONCLUSIONS
0.05% cyclosporine nanoemulsion type has simillar efficacy and subjectively less foreign body sensation.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Foulks GN, Lemp M, Jester J, et al. 2007 Report of the International Dry Eye WorkShop (DEWS). Ocul Surf. 2007; 5:65–204.2. Tatlipinar S, Akpek EK. Topical ciclosporin in the treatment of abdominal surface disorders. Br J Ophthalmol. 2005; 89:1363–7.3. Behrens A, Doyle JJ, Stern L, et al. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea. 2006; 25:900–7.4. Milner MS, Beckman KA, Luchs JI, et al. Dysfunctional tear abdominal: dry eye disease and associated tear film disorders – new strategies for diagnosis and treatment. Curr Opin Ophthalmol. 2017; 27(Suppl 1):3–47.5. Uchino M, Dogru M, Yagi Y, et al. The features of dry eye disease in a Japanese elderly population. Optom Vis Sci. 2006; 83:797–802.
Article6. Schaumberg DA, Dana R, Buring JE, Sullivan DA. Prevalence of dry eye disease among US men: estimates from the Physicians' Health Studies. Arch Ophthalmol. 2009; 127:763–8.7. Son DY, Hwang SS, Hyun J, et al. Prevalence and risk factors of dry eye disease after refractive surgery. J Korean Ophthalmol Soc. 2017; 58:782–7.
Article8. Donnenfeld E, Pflugfelder SC. Topical ophthalmic cyclosporine: pharmacology and clinical uses. Surv Ophthalmol. 2009; 54:321–38.9. Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II abdominal report. Ocul Surf. 2017; 15:438–510.10. Wan KH, Chen LJ, Young AL. Efficacy and safety of topical 0.05% cyclosporine eye drops in the treatment of dry eye syndrome: a abdominalatic review and meta-analysis. Ocul Surf. 2015; 13:213–25.11. Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000; 107:631–9.12. Sengupta P, Chatterjee B. Potential and future scope of nano-emulgel formulation for topical delivery of lipophilic drugs. Int J Pharm. 2017; 526:353–65.
Article13. Kymionis GD, Bouzoukis DI, Diakonis VF, Siganos C. Treatment of chronic dry eye: focus on cyclosporine. Clin Ophthalmol. 2008; 2:829–36.
Article14. Stonecipher KG, Torkildsen GL, Ousler GW 3rd, et al. The IMPACT study: a prospective evaluation of the effects of cyclosporine ophthalmic emulsion 0.05% on ocular surface staining and visual abdominal in patients with dry eye. Clin Ophthalmol. 2016; 10:887–95.15. Kale SN, Deore SL. Emulsion micro emulsion and nano emulsion: a review. Sys Rev Pharm. 2017; 8:39–47.
Article16. Anton N, Vandamme TF. Nano-emulsions and microemulsions: clarifications of the critical differences. Pharm Res. 2011; 28:978–85.
Article17. Khan W, Aldouby YH, Avramoff A, Domb AJ. Cyclosporin nano-sphere formulation for ophthalmic administration. Int J Pharm. 2012; 437:275–6.
Article18. Ayaki M, Yaguchi S, Iwasawa A, Koide R. Cytotoxicity of ophthalmic solutions with and without preservatives to human corneal endothelial cells, epithelial cells and conjunctival epithelial cells. Clin Exp Ophthalmol. 2008; 36:553–9.
Article19. Weiner ML, Salminen WF, Larson PR, et al. Toxicological review of inorganic phosphates. Food Chem Toxicol. 2001; 39:759–86.
Article20. Yang S, Byun YS, Rho CR, et al. Comparisons for evaluation of abdominal and safety of cyclosporin a 0.05% ophthalmic emulsion treatment groups. J Korean Ophthalmol Soc. 2016; 57:1849–56.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Efficacies and Safeties of a 0.05% Cyclosporine Nanoemulsion and a 0.1% Cyclosporine Cationic Emulsion
- Comparison of Absorption Profile between Microemulsion and Non-microemulsion Cyclosporine in Stable Renal Transplant Recipients and Therapeutic Drug Monitoring
- Contributing Factors Affecting Ocular Discomfort on Instillation and Compliance of 0.1% Cyclosporine A Cationic Nanoemulsion
- Efficacy of Topical Cyclosporine Nanoemulsion 0.05% Compared with Topical Cyclosporine Emulsion 0.05% and Diquafosol 3% in Dry Eye
- Comparison of Cyclosporine in Soft Gelatine Capsule and Microemulsion Cyclosporine in Renal Transplant Recipients