Electrolyte Blood Press.
2018 Dec;16(2):23-26. 10.5049/EBP.2018.16.2.23.
Long-term Tolvaptan Treatment of Autosomal Dominant Polycystic Kidney Disease in Korea
- Affiliations
-
- 1Department of Internal Medicine, Chonnam National University Medical School, Gwangju, Korea. skimw@chonnam.ac.kr
- 2Department of Radiology, Chonnam National University Medical School, Gwangju, Korea.
- 3Department of Internal Medicine, Saint Carollo Hospital, Suncheon, Jeollanam-do, Korea.
- KMID: 2439088
- DOI: http://doi.org/10.5049/EBP.2018.16.2.23
Abstract
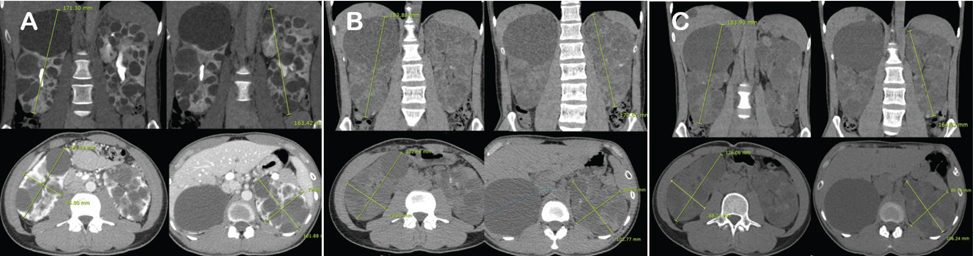
- A 22-year-old male patient was diagnosed with autosomal dominant polycystic kidney disease (ADPKD). He received conservative treatment with an angiotensin-converting enzyme inhibitor. Two years later, oral therapy, consisting of 60 mg tolvaptan per day, was initiated. Compared with height-adjusted total kidney volume, the rate of kidney growth reduced significantly from 7.33% to 0.66% annually, since commencement of the tolvaptan therapy. The liver enzyme profile and serum sodium level and osmolality were constantly within normal ranges. In Korea, this is the first reported case of a patient with ADPKD who received tolvaptan treatment for more than 1 year. This case demonstrates that long-term tolvaptan treatment appears to be safe, well tolerated, and effective for ADPKD.
MeSH Terms
Figure
Reference
-
1. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009; 150:604–612.
Article2. Gabow PA. Autosomal dominant polycystic kidney disease. Am J Kidney Dis. 1993; 22:511–512.
Article3. Gattone VH, 2nd , Wang X, Harris PC, Torres VE. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med. 2003; 9:1323–1326.
Article4. Gabow PA. Autosomal dominant polycystic kidney disease. N Engl J Med. 1993; 329:332–342.
Article5. Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007; 369:1287–1301.
Article6. Rieg T, Tang T, Murray F, et al. Adenylate cyclase 6 determines cAMP formation and aquaporin-2 phosphorylation and trafficking in inner medulla. J Am Soc Nephrol. 2010; 21:2059–2068.
Article7. Torres VE, Chapman AB, Devuyst O, et al. Investigators: Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012; 367:2407–2418.
Article8. Torres VE, Chapman AB, Devuyst O, et al. Investigators: Tolvaptan in Later-Stage Autosomal Dominant Polycystic Kidney Disease. N Engl J Med. 2017; 377:1930–1942.
Article9. Grantham JJ, Torres VE, Chapman AB, et al. Investigators: Volume progression in polycystic kidney disease. N Engl J Med. 2006; 354:2122–2130.10. Chapman AB, Bost JE, Torres VE, et al. Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2012; 7:479–486.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Tolvaptan: a possible preemptive treatment option in children with autosomal dominant polycystic kidney disease?
- Recent updates in therapeutic approach using tolvaptan for autosomal dominant polycystic kidney disease
- Management of autosomal dominant polycystic kidney disease in the era of disease-modifying treatment options
- Are you ready to accompany autosomal dominant polycystic kidney disease patients in their treatment journey? Real practice for selecting rapid progressors and treatment with tolvaptan
- A Case of Renal Cell Carcinoma in Autosomal Dominant Polycystic Kidney Disease Hemodialyzed